Black-crowned Night-Heron
Nycticorax nycticorax (Linnaeus)
Ardea nycticorax Linnaeus, 1758. Syst. Nat. ed. 10(1), p. 143: southern Europe.
Subspecies: Nycticorax nycticorax obscurus Bonaparte, 1857: Chile and Patagonia.
Other names: Night Heron, Black Capped Night Heron, Quawk, Quok, Crabcracker in English; Martinete, Martinete Común Perro de Agua, Guaco, Garza Bruja, Huairava, Boca d’agua Rey Congo, Yaboa Real, Guanabá, Guanabá de la Florida in Spanish; Savacu in Portuguese; Bilhoreau gris, Héron bilhoreau, Coq de nuit, Coq d'eu, Crabier bois, Crabier grosse tête Bilhoreau a couronne noire in French; Nachtreiher in German; Kwak in Dutch; Кваква in Russian; Natthäger in Swedish; Goi-sagi in Japanese; Lapay in Pilipino (Philippines); Kowak in Indonesian; Ye lu in Chinese.
Description
The Black-crowned Night-Heron is a stocky dark grey and white heron with a distinctive glossy black crown and back.
Adult: The Black-crowned Night-Heron has black cap that goes forward to a white line above the bill. The sides of the head and thick neck are white. The thick, down curved bill is black. The lores are green blue; the irises are crimson red. The back is black, and upper wings, rump and tail are grey. The belly is white to pale grey. The relatively short legs and feet are pale yellow. During courtship, the lores are black and legs and feet are red to pink. The black plumage of the head and back takes on a blue green gloss and white head plumes develop that may reach a length of 25 cm.
Variation: Females average smaller than males in most measurements and have shorter head plumes during the breeding season. Black-crowned Night-Herons vary in size and color geographically and up to four races have been recognized on these bases. However, variation among individuals is high, light, dark and intermediate color birds occur in South America, with very dark and cream colored birds have been reported (Pitelka 1938, Gochfeld et al. 1982, Davis 1993). Obscurus is larger with a slate grey back and brown grey face and chest. Given its extraordinary range it is intriguing that more geographic variation is not recognizable. Other subspecies, hoactli and falklandicus, have been described but are doubtful.
Juvenile: Juveniles have brown plumage, very different from that of the adults (McVaugh 1972). The head and upper parts are grey brown with buff, white, or rufous spots. Lores are green and the irises are orange yellow to brown red, changing to red at 2-3 years. The stout bill is dark and horn. The upper bill is black with yellow or green sides, becoming black with green sides at one year. The lower bill is horn, turning yellow with horn tip or yellow green with black tip about 1 year, and black by 2 years. Upper wing is grey brown with lighter spots; flight feathers are grey brown with white tips. Upper tail coverts brown. Rump is grey brown streaked with white. Tail is grey. Under parts are grey with dark brown streaks. The legs are yellow green to olive green, turning yellow by 2 years. By the age of one year, the juvenile is still has a brown wash, brown cap and back, with some spots remaining and striped below (Davis 1999). Older juveniles (2-3 years) gradually take on adult characters, becoming more solidly dark above with the spots disappearing and lighter below, with some remnant brown feathers persisting on the head, back or wings.
Chick: The chick has buff brown down on its back, light down below. The outer portions of the crown down are white creating a crest. The bill is pink with dark tip. Iris is gray olive, changing to light yellow, and then bright yellow by 30 days. Lores are grey at hatching becoming grey green. Legs and feet are brown at hatching, turning yellow olive, then olive grey, and finally light green at 30 days.
Voice: The Black-crowned Night-Heron is a noisy, quarrelsome bird. Its typical call gives it similar colloquial names around the word - Quawk, Quok, Coq, Kwak, Kowak. This “Quok” call is a hoarse raucous flight call. The “Plup” call is the advertising call. This is a unique call in which the sound of a rubber band being plunked is followed by a buzz or hiss, rendered “snap-hss” or “plup-bzz”. In addition to its advertising function, it may also serve a group coherence function (Albert and Wollesmann 1986). “Rok” call, rendered “rok, rok” is the threat call. “Wok” call, rendered “wok, wok” is used for disturbance, in the Greeting Ceremony, and during precopulation. “Kak” call, rendered “kak, kak” is the landing call. Young beg with “yip, yip, yip” becoming “yak, yak, yak”, and finally “chuck, chuck, chuck” (Davis 1993). Birds may use a bill rattle in copulation and greeting.
Weights and measurements: Length: 58-65 cm; Weight: 727-1,014 g.
Field characters
The Black-crowned Night-Heron is a black, white and grey bird, identified by its black cap and back, white neck and grey wings, stocky build, and when breeding white head plumes. In flight, it has a distinctive compressed outline with broad, round wings. The wing beats are faster than most herons; its toes only barely extend beyond its tail, and it often gives its typical Quok calls while flying.
It is distinguished from the Rufous Night-Heron by its black (not chestnut) upperparts. It is distinguished from the Yellow-crown Night-Heron by its black (not yellow) crown and three toned (not grey) body. It is distinguished from the large Ardea by its smaller size, shorter thicker neck, relatively short legs, haunched posture, black back, and all grey wings in flight.
The juvenile looks like other herons with cryptic plumage. It is distinguished from the juvenile Yellow-crowned Night-Heron by having buff white spots, darker ventral streaking, narrower, longer bill, thicker neck, more crouched posture, foot tips (not feet) projecting beyond the tail in flight, and lower pitched call. It is distinguished from the several large bitterns whose ranges it overlaps, by prominent white spotting on the back, smaller, brown (not gold brown) color, spotted pale brown (not dark brown) wings, lack of black face markings, taller stance, in flight having toes (but not feet) projecting beyond the tail and rapid (not slow) wing beats. It is distinguished only with difficulty from the immature Rufous Night-Heron by its grey brown (not rufous brown) base color, grey brown white tipped flight feathers (not rufous brown with dark brown inner edges), brown (not dark brown) upper tail coverts, grey brown (not white) rump, and grey (not rufous brown) tail.
Systematics
The Black-crowned Night-Heron is closely related to the Rufous Night-Heron, its Australasian counterpart. They have hybridized where their ranges have come into contact in Java and Borneo (Hubbard 1976, Sheldon and Marin 1984). They also now overlap in the Philippines and in the past in Celebes (White 1973). Field observations did not indicate inter-nesting. Additional observations in the field are needed. Patterns of geographic variation among Nycticorax nycticorax populations are not clear. Particularly unclear is the status and ranges of the two subspecies occurring in South America. Intraspecific geographic variation deserves additional study, as does the relationships among the Black-crowned and Rufous Night-Herons and the several now extinct island night herons from Reunion (dubosi), Mauritius (megacephalus), and Rodrigues (megacephalus) (Hilton-Taylor 2000).
Range and status
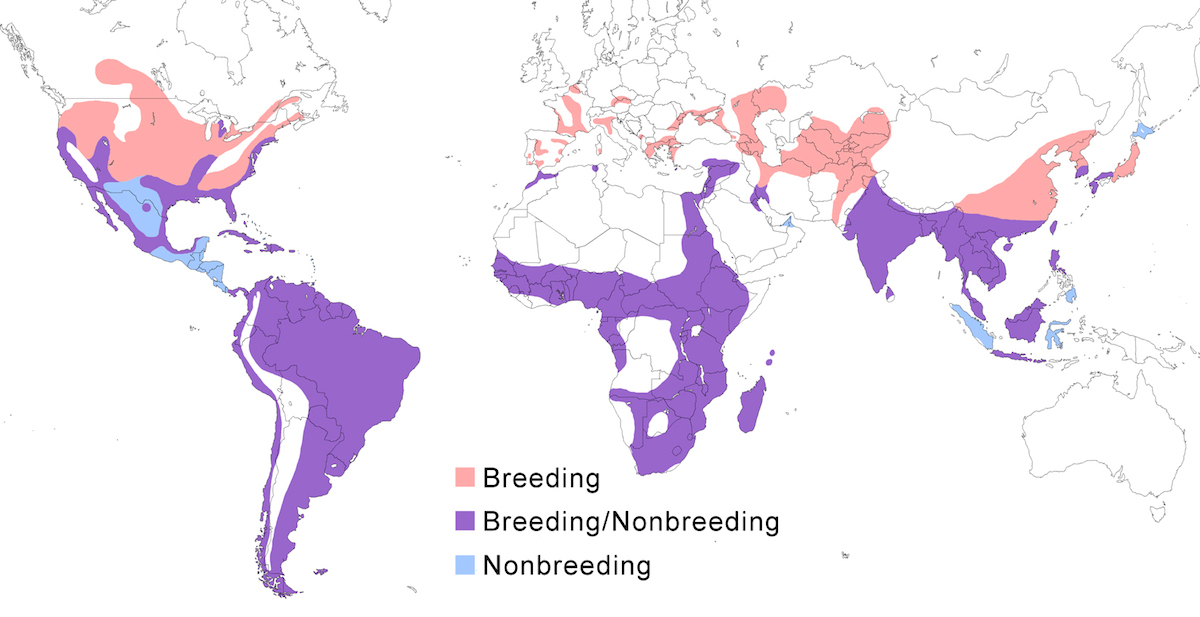
The Black-crowned Night-Heron occurs across the temperate and tropical world from North and South America, Europe, Africa, and Asia to the East Indies.
Breeding range: The subspecies obscurus occurs south of the Atacama Desert in Chile and Rio Negro in Argentina, and is found as far south and east as Tierra del Fuego and the Falklands. Its northern and western range boundaries are not clear and require additional study.
The subspecies nycticorax, breeds in North America, nesting in Canada (British Columbia, Alberta, Saskatchewan, Manitoba), west United States (Washington, south Idaho, Montana), mid United States (south Minnesota, south Wisconsin, Illinois, Ohio), and east Canada (Ontario, Quebec, New Brunswick), south through the rest of United States except mountains, the Greater Antilles, larger Bahama Islands, Cayman Island, to the Virgin Islands, and coastal Mexico (to Jalisco and Campeche). It nests in Panama and into South America. It is now known to breed in the Amazon and high Andes of Peru through Chile (Morales 2000), to Argentina. It is also found in the Galapagos and Hawaii.
In Europe, it nests in Spain, Portugal, France, Belgium, Netherlands (Verbruggen 1989, Eerhert and Kurstjens 2000), Germany, Austria, Czech Republic. Slovakia, Poland, Ukraine, Belarus (Samusenko and Pinchuk 1999), Moldova, Russia, Romania, Bulgaria, Hungary, Italy, Slovenia, Croatia, Albania, Greece, and Turkey (Marion et al. 2000).
In Africa, it breeds in Nigeria, Central African Republic, DR Congo, northwest Angola, Tanzania, Malawi, Zimbabwe, Botswana, Namibia, South Africa, Madagascar, and Seychelles.
In Asia, it occurs in places in the Middle east and extends more continuously across the continent, nesting in Israel, Saudi Arabia (James 1991), United Arab Emirates, Iran, Azerbaijan, Turkmenistan (Eminov 1993), Uzbekistan, Kazakhstan, Kyrgystan, Tajikistan, Russia (Komarov and Lipkovich 1986), Afghanistan, Pakistan, India, Nepal, Sri Lanka, Bangladesh, Myanmar, Cambodia, Laos, Vietnam, China (south east Sichuan, Henan (Zhang et al. 1994). Hunan, Guangxi, Guangdong, Zhejiang, Hong Kong, Taiwan), south Korea (Yu and Hahm 1997), Japan, Philippines (Luzon, probably also Mindanao and other islands — R. Lansdown pers. comm.), Malaysia (single colony on west coast of peninsula — R. Lansdown pers. comm., Singapore, Brunei, Indonesia (to Borneo, Java).
Nonbreeding range: Temperate populations tend to be partially migratory, and tropical and subtropical populations tend to be more sedentary, although dispersal occurs in both. Throughout its northern hemisphere range, the Black-crowned Night-Heron winters fairly far north.
In North American populations are partially migratory. Some remain as far north as south Oregon, Great Lakes and south Maine, but most winter in the southern United States (south California, south Arizona, south Texas, to Florida), the Mississippi valley, West Indies through the Lesser Antilles, especially Cuba, west and east Mexican coast, central Mexico, and Central America.
European populations also are partially migratory. Some remain in Europe along the Mediterranean coast and even inland from Spain to Greece north to Ukraine (Rusev 1984), and also Israel and Morocco (Tosi and Toso 1979, Ham 1986, Barbieri and Fasola 1986, Brugiere 1999, Hafner 2000). Most European birds winter in tropical Africa, ranging at least to Mozambique (Fasola et al. 2000). Herons breeding in Africa are mostly sedentary, but some South African herons disperse north to DR Congo and Mozambique (Harrison et al. 1997). Others winter in Iran, Iraq, Pakistan (especially Sind), India (especially Tamil Nadu), Maldives, south east China, south Korea (Kim et al. 1997), Japan, Philippines and Indonesia. Southern Asia birds are sedentary.
Migration: In the Black-crowned Night-Heron, post breeding dispersal merges into migration. Birds migrate at night alone or in small groups. In North America eastern birds move southward in late September and October along the Atlantic coast, down the Mississippi Valley, and along the Pacific coast. Birds overlap broadly in destination: Florida, Gulf Coast, coastal Mexico, central Mexico, Central America and the West Indies. Return is early, mid March–mid May (Byrd 1978, L’arrivee and Blokpoel 1990). Similar movements may take place in South America, but information is not complete. In Europe southward migration is in August. Broad front movements cross the Mediterranean and Sahara to tropical Africa. They return to the breeding grounds in March–May. East Europe and west Asia birds move south to Iran, Iraq, west and central tropical Africa, and to Pakistan and India. In West Africa, herons are sedentary; in southern Africa some migrate north. In east Asia, northern populations are migratory, southern populations are sedentary. West Asian birds move south to southeast China and Japan and then to the Philippines and Indonesia.
The Black-crowned Night-Herons, especially juveniles, disperse widely after nesting is completed and also over shoot their return to the breeding grounds on return migration. These movements bring them to casual or even regular occurrences beyond the breeding range, such as Alaska (Gibson et al. 1987b), Newfoundland, Greenland, Iceland, England, Ireland, Micronesia, Wake Island, the Galapagos, the Azores, Sumatra, Cocos-Neeling, North Mariana, Guam. Individuals wander widely from their natal colony. French bred birds were found later in Romania and in Russia. This dispersal is an important factor inhibiting geographic isolation among populations.
Status: The Black-crowned Night-Heron is widespread and abundant locally throughout most of its range. In Europe, populations are increasing following both population and range decreases in late 19th century due to habitat destruction and hunting (Fasola and Hudec 1997, Marion 1997). The European population now is 50,000-75,000 pairs, concentrated in Italy, which now has 14,000-24,000 pairs, and in Russia with 10,000-15,000 pairs (Marion et al. 2000). About 1,000 pairs nest along the Mediterranean coast in France, 2,200 pairs in Spain, and 1-200 pairs in Portugal (Hafner 2000). In some areas such as Netherlands, Germany, and Belgium, colonies established artificially aided in the recovery. In other areas (Italy) development of rice field cultivation supported the population increase. Populations fluctuate but are stable or increasing overall in Europe. Regionally they appear to be decreasing in Spain, Mediterranean France, Croatia (500 pairs), and Romania (Danube Delta decreased 47% to 3,100 pairs in mid 1980's) (Hafner 2000). The wintering population in tropical Africa is estimated to be 70,000-100,000 birds, including 10,000 at the Senegal Delta. Conditions in Africa are critical in that 95% of European herons winter south of the Sahara and a high proportion of European juveniles may remain there 2 to 3 years.
The African breeding population is common and widespread in sub-Saharan Africa with colonies in the hundreds to low thousands (Turner 2000). In 1962 a single colony in Tanzania was counted to have 1000 pairs and it remains common in that country (Baker and Baker in prep.) It is rarer across north Africa, with small numbers breeding (and expanding their range inland) in Algeria, and 500-1,500 pairs in Morocco. It is also fairly common on Madagascar. Overall the resident population in tropical Africa (including Madagascar and Seychelles) is estimated to be between 25,000 and 100,000 birds (Rose and Scott 1994).
In the Middle East, Israel has 2,000 pairs nesting (Paz 1987) and the species appears to be increasing its range including in the Arabian Peninsula using artificial wetlands (Perennou et al. 2000). In central and east Asia, the species is common and widespread, although difficult to census. It is increasing in Malaysia (one area increasing from 4,000 to 12,000 birds in twenty years to the mid 1980's). Colonies numbering in the thousands have been reported from India, Pakistan, Malaysia, Indonesia (Java), China, and Japan. In central China, partial censuses in 2000-2001 located 10 heronries with total 22,500 Black-crowned Night-Heron nests in a 2,000 km2 area near Lake Poyang, and 5 heronries with 45,700 nests along 200 km transect near Lake Tai (Fasola pers. comm.).
In North America, the species’ range has been stable, although regional population changes may be occurring—increasing in the northeast and decreasing in mid-continent and mid Atlantic states (Butler et al. 2000). In 1970’s over 48,000 birds were censused in part of the United States (Spendelow and Patton 1988). The population is increasing in Hawaii (Engilis and Pratt 1993). It is common and abundant in South America, with 50,000-250,000 pairs estimated in Venezuela (Morales 2000).
Habitat
The Black-crowned Night-Heron has wide habitat preferences. It occurs most frequently on the vegetated margins of shallow freshwater or brackish rivers, streams, ponds, lakes, marshes, swamps, mangroves, and mud flats. It often feeds from on top of aquatic vegetation, for example on kelp beds 500 m offshore in the Falklands. It also uses grasslands and coastal habitats, especially on migration, and high mountains, nesting to 4,800 m in Chile — exceptional for a heron. The species frequently, perhaps characteristically, uses human-made habitats. These include pastures, ponds, reservoirs, canals, ditches, fish ponds, rice fields, wet crop fields, and dry grasslands. Rice fields are especially important habitats in much of its range (Fasola et al. 1996). In areas where rice fields are available, up to 96% of the food resources of a colony were taken from that habitat (Fasola and Ruiz 1996, Fasola et al. 1991,1993).
Despite the wide range of habitat use, large nesting colonies seem to be associated with protected sites in large wetlands (Alieri et al. 1988, Hafner and Fasola 1992, Davis 1993). Colonies are on islands protected from predators and often—although not necessarily—away from human disturbance.
Since night herons rest during the day, roosts become particularly important habitat features. The heron is flexible in its choice of roosting substrates. It uses trees, bamboo, pines, tamarisks, mangroves, oaks, jacarandas, drowned trees, and mixed wood lots. Roosts need not be over water, and often are in parks and other human environments. Habitat essentials appear to be dense roosting cover, not necessarily free from human disturbance, and fresh, brackish or saltwater feeding grounds nearby.
Foraging
The foraging behavior and food habits of this species are well studied (Drinkwater 1958, Wolford and Boag 1971, Kushlan 1973, Watmough 1978, Parasharya 1982, Sodhi 1985, Voisin 1991, Fasola and Ghidini 1983, Fasola 1984, 1994, Fasola et al. 1993, Prigioni et al. 1985, Yen 1991, Davis 1993, Fasola and Hafner 1997, Boukhemza et al. 2000). Black-crowned Night-Herons typically feed by Standing intermittently mixed with Walking Slowly. They Stand in a Crouched posture and carefully stare at the water until seeing or hearing something and then make a lunging strike. Herons tend to switch from Standing to Walking when the feeding success on smaller prey increases (Fasola 1984).
This is a nocturnal species and most often and typically feed at night, locating prey by sight and by sound. But they do readily feed during the day and are required to do so to meet the energy demands of raising young (Fasola 1984, Endo and Sawara 2000). It has relatively large and more widely separated eyes than do the more diurnal herons, which is likely associated with visual ability over a range of natural light levels (Katzir and Martin 1998). The wide placement of the eyes does not, however, have any better binocular vision. Feeding behavior is the same day or night, but efficiency is less during the day in that they catch fewer, although larger, prey (Watmough 1978). Daylight also allows use of more active behaviors such as Running, Bill Vibrating, Hovering, Feet First Diving, Swimming Feeding, Plunging, and Underwing Feeding. Along with very few other herons, this species is known to use Baiting (Davis and Zickefoose 1998). Daylight feeding brings it into conflict with typically day feeding herons. During the day they are displaced and robbed by other herons, and appear to be definitely subordinate to the day feeding herons (Kushlan 1973, 1978a, Watmough 1978).
The Black-crowned Night-Heron is mostly a solitary forager. It usually feeds alone, maintaining exclusive feeding territories. In defense it uses Forward, Supplanting Runs and Supplanting Flight. It more often attacks other night herons than other species (Fasola 1986). It feeds in loose, dispersed aggregations and also in denser aggregations when prey is highly concentrated (Fasola 1982). These are found by social interaction, following birds flying to feed and also joining birds already feeding at a site (Fasola 1982).
They roost during the day, except during breeding, either alone or communally in groups of 10 to a hundred birds (Perlmutter 1992, Igarashi 1996, 1997). They prefer to roost in dense trees, presumably for protection. They fly out of the roosts at dusk, 10-20 minutes after sunset, heading to feeding grounds, and giving their characteristic Quok call. They return to roost the following morning, averaging 17 minutes before sunrise (Fasola 1984). Time budgets are constrained by season (Kim et al. 1997).
The food habits of this heron have been well documented. Insects, frogs, and fish dominate the diet. A diet might include all three types of prey, having different relative importance in different areas or at different times. For example in one study, in ricefields amphibians predominated whereas in rivers and lagoons, fish predominated. The overall food list is quite large, given that the night herons are opportunistic foragers and will eat what they encounter
Insects include beetles, bugs, grasshoppers, crickets, flies, dragonflies. Other invertebrates include spiders, leeches (Hirudo), earthworms, prawns, crabs (Uca), crayfish (Cambarus), mussels (Mytilus), clams (Venus), and squid (Loligo). It also eats carrion (Parejo 1997).
Many types of fish are taken including carp (Cyprinus, Carassius), cichlid (Tilapia), mosquitofish (Gambusia), perch (Perca), roch (Rutilus), tench (Tinca), herrings (Clupeidae), suckers (Catostomidae), minnows (Cyprinidae), killifish (Cyprinodontidae), eel (Anguilla), barbel (Barbus), catfish (Ictaluris), stickleback (Gasterosteidae), pickerels (Esox), sunfish (Lepomis), mullet (Mugil, Liza), flounder, sleeper (Dormitator) and many more (Davis 1993, Fasola and Hafner 1997). Amphibians include frogs and toads (Rana, Bufo, Pseudacris, Hyla) and newts (Ambystoma, Triton) (dead or alive). Other foods include lizards (Lacerta), snakes (Thamnophis, Natrix), rodents (Rattus, Apodemis, Microtus), shrews (Sorex), bats, birds and their eggs.
The propensity of the Black-crowned Night-Heron to eat birds is unusual among herons, may constitute an important part of the diet of some individuals. This species often take young of other colonial nesting waterbirds such as terns, herons and ibises, which it obtains by walking though the colony. In eastern North America, reports include nestling White Ibis (Eudocimus), Cattle Egrets, and Great Egrets. It also eats noncolonial marshbirds such as stilts (Himantopus) and duck (Anas). In one example, a heron perched on nest boxes and ate swallows (Tachycineta) as they emerged (Miller 1988-89).
Breeding
The courtship behavior and breeding biology have been well studied, including being the object of some of the classical studies in behavior (Lorenz 1938, Noble et al. 1938, Allen and Mangels 1940, Palmer 1962, and Voisin 1970, 1991, Chapman et al. 1981, Custer 1991, Custer et al. 1983a, b, 1984, Prigioni et al. 1985, Alvarado 1986, Custer and Frederick 1990, Custer and Peterson 1991, Erwin et al. 1996b, Blus et al. 1997, Yu and Hahm 1997, Voskamp and Zoetebier 1999, Zhu et al. 2000).
The nesting season differs geographically. In temperate areas, it is in the local spring, often early, but in tropical and subtropical areas nesting is variable and generally coinciding with the rainy season. Breeding takes place in April–June in Canada, March–July in east United States, July–August in Costa Rica, April–June in southern Europe and North Africa, April and August–September in Ethiopia, December–January and again April–June in East Africa, November–May in DR Congo, January–April in Zambia, August–March peaking September–January in southern Africa, August–January peaking November–December in Madagascar, December–April in East Java, February–July in West Java.
Nesting habitat is highly variable. It nests usually in bushes and trees of many species, up to 50 m tall, but also in reeds, sedge, grass tussocks, and on the ground (Alvarado 1986, Davis 1993, Kobayashi 2000). Colony sites are reused in most places; however, in other places, sites used change from year to year (Subramanya 1996). Colony site movement can be a response to birds destroying their trees through nesting activities. The species often nests in rural, suburban and urban settings. Across its range, it seems to be particularly attracted to nesting in zoos, some of which began as captive colonies and continued as birds became feral.
Nesting is usually colonial, in single species colonies or in mixed species colonies with other herons, cormorants (Phalacrocorax), or ibis (Eudocimus, Plegadis). Nests may total in the thousands, but the night heron usually remains in discrete single-species groups within the larger colony. They tend to nest very close to each other, with as many as 20-30 nests in one tree. Colonies are dispersed on the landscape in relation to distance from feeding areas.
Nests are a platform of sticks and reeds, 30-45 cm wide and 20-30 cm high. The nests start as fragile structures, which, however, are added to through incubation. Nests that survive are reused, enlarged, and become quite bulky. In one study, 86% of birds used old nests (Davis 1986a). Males collect the twigs and present them to the females for insertion into the nest. Sticks are often stolen from other nests.
The male chooses a site from which to display, often an old nest. During courtship the display plumes are in full development, and the white head plumes are particularly featured. The male claims its site and defends it using Standing, Preening, Upright, and Forward. In the Upright threat, the head and neck is up and forward, and crown, neck and back feathers partially erect, gives the Rok call. In full display the Forward may involve maximum erection of feathers and head plumes, the eyes bulge out, and the Rok call is given. The night heron will attack with minimal previous display, launching a Supplanting Flight with strong blows of the bill. Similarly, it will engage in Face to Face Fighting, with one bird striking or grabbing the bill or wing of the opponent.
The Snap display (also called bowing display) in this species is performed in a crouch, head is lowered and extended, feathers of head, back and neck partially erected, and holding a twig in the bill, or they snap the bill. Unmated males use it as an advertisement display.
The most common advertising display is the Stretch-snap, a unique display derived from the Stretch (Voisin 1991) that has also been called reverse stretch, foot-lifting, snap-hiss, song-and-dance, and danse (in French). The bird stands erect, treading from one foot to the other (or lifts one foot repeatedly), extends its head and neck forward and down, feathers are not raised, and the eyes bulge. With its bill down, it gives Plop calls, which can go on for many minutes. Between songs it does Preening to its belly and Twig Shaking. After the Stretch-snap, a bird may lift its head upwards with the bill pointing to the sky, in a more typical Stretch display.
The males also do a Twig Shake display as part of pair formation. The female manages to enter and maintain herself in the male’s territory to form the pair. After pair formation, Wing Touch, Bill Clappering, Billing, and Back Biting are all performed to establish and maintain the pair.
In the Greeting Ceremony both sexes bend horizontally with outstretched neck, head plumes raised, and touch bills, which they may rattle together, and also give the Wok call. Analysis of this display originates with Conrad Lorenz (1938), but it is still unclear if individual recognition is possible from the call (Venchi et al. 1994). The twig-holding Stretch display is also used in greeting and in twig passing, and Billing and Back Biting also commonly attend nest relief. Both birds actively defend the nest site. Copulation is not preceded by a set display, but may follow a Greeting Ceremony.
The eggs are green to pale blue-green (Custer 1991). They average 50 x 36 mm in Europe, 52.3 x 37.37 mm in North America (Davis 1993), 51.35 x 37.43 in Central America (Alvarado 1986), 48.0 x 34.3 in China (Zhang et al. 1994), 49 x 35 in Africa (Brown et al.1982), 50.9 x 35.8 in Madagascar. Clutch is usually about 2-5 eggs (Custer et al. 1983a), range is 1-7 eggs. Averages include 4.5 north east North America, 3.9 west North America, 3.6 inland North America, 2 Falklands, 3.3 East Africa, 2.8 Zimbabwe, 2.2-2.4 South Africa, 2.5 in southern France, 2.6 in northern Italy, 4.1 in Korea, 3.55 and 3.49 in China. Eggs are laid at two-day intervals. They generally will have only one nest per season, but will readily produce additional clutches if the first nest fails (Blus et al. 1997).
Incubation is continuous, starting with the first egg. Both parents incubate and attend the nest. Incubation averages about 23 days, with a range of 21-26 days. Eggs hatch asynchronously. At hatching young are semialtricial and nidicolous. Hatching success is fairly high, 75% in China (Zhang et al. 2000). Both parents attend the nest and brood the young for 10 days after hatching. They are then left increasingly alone, and are not guarded at all after 20 days. Adults feed the young by regurgitation; the young grab the adult’s bill and force the regurgitation. Later the adults put the food on the nest floor. They feed about 7 times per day.
The appendages develop rapidly (Chapman et al. 1981, Galeotti 1982). Temperature is regulated by day 10 and the young begin clambering out of the nest by three weeks. At 35 days, they are fully feathered. They fledge in 6-7 weeks. For much of the time they are extremely noisy, constantly enlarging their repertoire from the high-pitched cackle of their first two weeks. Brood reduction is common, especially for the last hatched chick. Growth is slower for the third chick but there is little evidence of chicks killing siblings (Custer and Peterson 1991, Emond and Medeiros 1999, Medeiros et al. 2000). Chick death appears to occur predominantly in the first few days after hatching (Prigioni et al. 1985). Nesting success (young per egg) is often high, 75-85% in North America (Henny 1972), 70% in France (Hafner 1978). Production is variable, 0.47-1.94 young per successful nest in studies in North America (Blus et al. 1997), 2.08 from 58 successful broods in East Africa (Brown et al. 1982), and up to 2.9 in Central China (M. Fasola pers. comm.). Food supply, drought, storms and predators reduce production. Predators of eggs and young include raccoons (Procyon), muskrats (Ondatra), owls (Bubo), gulls (Larus), caracara (Polyoborus), crows (Corvus), jays (Cyanocitta), lizards (Iguana), and snakes (Boa).
Population dynamics
Most individuals nest at two to three years old, but yearlings sometimes breed (Custer and Davis 1982). In central China, 41% of the breeders are birds in juvenile plumage, probably in their second year. Most of the pairs are strictly assorted by age, and the reproductive success of pairs of adults is higher than the success of pairs of juveniles (Fasola et al. 2001).
Mortality rate in the first year may be high, up to 69% (Henny 1972), but decreases later. One longevity record of 16 1/2 years has been reported (Houston 1974). Juveniles from Europe depend on African habitats for up to several years, so it would be expected that conditions there could affect nesting populations; 10-20% of the population variability is explained by African climate (Fasola et al. 2000).
Conservation
Although widespread across one of the most cosmopolitan ranges among herons, the Black-crowned Night-Heron’s nesting is limited to a few areas in most regions. As a result conservation of these sites becomes crucial. The protection of nesting sites and the management of feeding sites are both required to ensure regional population stability. One successful conservation scheme, in Italy, involved the protection of existing colony sites, the active management of habitat, and the creation of a network of new breeding sites spaced appropriately (Fasola and Alieri 1992b, Fasola et al. 1992). Colony and roost site management takes a landscape perspective. Colony sites are destroyed by the bird’s activities, and so alternative sites need to be available. Colony impacts are only important if there are no other sites to move to (Marion et al. 2000). They readily nest on artificial sites, such as spoil islands, prepared for them. The attraction and accommodation of Black-crowned Night-Herons to zoos and other urban settings provide many opportunities for environmental education. Zoos within the range of the species should be encouraged to establish nesting sites for wild populations (of the local genetic stock) that can be managed for their education value. The flexibility of this species allows options management of colony sites at massive scale (Crouch et al. 2002).
The dependence of regional populations on large wetlands for both breeding and non-breeding, places the conservation of the species in the full context of wetland conservation. Wetland drainage historically undermined their population stability in Europe. Wintering sites on the Mesopotamian marshes in Iraq are threatened by massive development schemes. Large wetland ecosystems will need to be protected and managed for night herons to maintain stable regional populations. Wetland drainage and the destruction of vegetation has led to a decline on Black-crowned Night-Herons in many parts of Africa (D. Turner pers. comm.). It is only in Zimbabwe and South Africa that through the provision of artificial water bodies and the planting of trees are there signs of an increase in both the range and numbers of this species.
Other human interactions must also be considered in developing conservation strategies for this species. In North America, populations declined due to pesticides, particularly up to the 1960’s. Since then populations have recovered, contaminant loading has decreased, and present contaminant burdens tend to be minor or not proven to be of significant populational consequence (Henny et al. 1985, Hothem et al. 1995, Movalli et al. 1995, Hoffman et al. 1986, Blus et al. 1997, Fasola et al. 1998, Rattner et al. 2000). Chronic contamination can still occur where persistent pesticides are still being applied (Henny and Blus 1986). It has been thought for some time that high contaminant levels in herons from the western USA were due to accumulation in winter in Mexico, but careful reexamination suggests that data supporting this view are slim (Mora 1997). Herons also are at risk from poisoning by lethal materials (Hunt et al. 1995). Monitoring of contaminant burdens in this species is a good environmental indication of contaminant availability in breeding or wintering areas (Custer et al. 1991, Custer and Custer 1995). Interactions with aquaculture and agriculture also need to be considered. Herons traditionally have been shot at fish hatcheries, although they are less seen and obvious when they feed at night. The true impact of the species at aquaculture facilities needs to be determined and conservation-friendly measures employed (Ashkenazi and Yom-Tov 1996). Beyond antidepredation killing, hunting mortality continues. In some areas, like Madagascar, herons are still taken for food. Rice fields support some of the largest populations, and the culture techniques conducive to these herons need to be continued. Black-crowned Night-Herons are fairly tolerant of non-aggressive human activities, and often nest and roost near humans. This can bring them into conflict with towns that would prefer not to have colonies and roosts nearby. The management of sites and public education are required. Local disturbance, especially at egg laying, can provoke abandonment of the nests (Tremblay and Ellison 1979, Parsons and Burger 1982, Marion et al. 2000).
Research needs
A primary research and conservation need is to unravel the patterns and taxonomic consequences of geographic and species variation in the Nycticorax herons. The proper allocation of populations to species and to subspecies requires a re-examination of Nycticorax nycticorax, N. caledonicus, and N. leuconotus as well as population variation within N. nycticorax using plumage, morphometric, and genetic characters. Also, the now extinct island populations of Nycticorax should be examined from the larger perspective of species and subspecies limits in the genus. The breeding biology and ecology of the species are well known. Intriguing questions remain however. One is its apparent subservient position among herons during daytime foraging, which seems unexpected for such an aggressive heron. The question might be cast within the relative costs and benefits of aggressive behaviour during day and night. Prey capture at night is not completely understood, and studies of the physiology and behaviour of night foraging may prove interesting. Conservation research is needed to determine the best approaches to landscape-based population protection and management. Successes in Italy suggest ways that colony site protection, creation, and habitat preservation can be used to create a regional conservation strategy. This model can be tested elsewhere. The status of the species throughout its range needs to be better understood. The recent discovery of large colonies in China is an example of how little is actually understood about the status and trends of this widespread species.
Overview
The core of the Black-crowned Night-Heron’s ecology is its adaptation to nocturnality. Night-time foraging limits the options of suitable foraging behaviour. As a result the heron is primarily a stand and wait predator. It intermixes slow Walking to shift locations, to investigate a potential prey, or to cover more ground. Its two behaviours produce different results, with Walking being better for small abundant prey and Standing better for larger prey. The heron defends it feeding site and only forages communally when prey abundance is high. It is morphologically designed for its slow foraging. It has relatively short legs and neck for standing in a crouched posture close to the water. It has a plumage that is cryptic at night, light below from the fishes’ view and dark above from a predator or other heron’s view. It has huge eyes to see better under a variety of conditions. Its daily schedule is to feed at night and sleep during the day. The nocturnal habit of the species must, however, break down during nesting. As it is unable to secure sufficient food for itself and its young foraging only at night, so it must feed during the day, on larger, scarcer prey in competition with the day-feeding herons, to which it is subordinate. It sometimes resorts to active behaviours under these conditions. Dense food supplies or high availability are essential for the heron to meet the energy demands of nesting. One place of high availability is the colony itself where it eats other birds. Its habitat choice is broad, but it clearly depends on large natural wetlands and on artificially provided situations such as rice fields and fish ponds, on which it has become dependent. The loss of habitat in Europe led to a population collapse, and the creation of fish farms, rice fields, and polders brought it back. It similarly is able to use human-provided nesting sites. So, it has adopted living in close proximity to humans. It has three separate lines of food: fish, insects, and frogs, which can vary in importance depending on their availability. It is a species that takes advantage of a varied and changeable array of food––a food generalist. The Black-crowned Night-Heron is adaptable not only in food and nesting, but in its travels. A strong dispersal tendency brings it to places outside the nesting range, which can ensure colonization of likely locations. The species, and its close relative N. caledonicus, together cover much of the world.