Cattle Egret
Ardea ibis (Linnaeus)
Ardea ibis Linnaeus 1758. Syst. Nat. ed. 10, 144: Egypt.
Subspecies: Ardea ibis coromanda (Boddaert), 1783: Coromandel.
Other names: Buff-backed Heron, Cow Crane, Cow Bird, Cow Heron, Elephant Bird, Tick Bird in English; Garilla Bueyera, Garza del Ganado, Garza Ganadera in Spanish; Garça vaqueira in Portuguese; Héron garde-boeufs in French; Kuhreiher in German; Koereiger in Dutch; Kohäger in Swedish; Египетская цапля in Russian; Kuntul kerbau in Indonesian, Talud in Pilipino, Taga kalbao in Tagalog (Philippines); Ama-sagi in Japanese; Da Bailu in Chinese.
Description
The Cattle Egret is a short-legged white egret, with a relatively short yellow bill, usually seen feeding in flocks in grasslands and pastures following cattle or perched in a haunched posture.
Adult: The Cattle Egret is a basically white bird. The bill, lores, and irises are yellow. Some nonbreeding birds have buff back or chest plumes, which some individuals tend to keep after nesting is completed. Legs are green to black, light near the leg feathering.
In breeding season, they develop long rufous buff plumes on the crown, back and lower neck (Siefried 1971c). The extent of these plumes differs between subspecies. In courtship the bill, legs, and irises become bright red and the lores turn purple pink. At the peak of the courting period, the skin becomes a bright cobalt blue color (Lancaster 1970, Maddock 1993). This red color is lost quickly after mating. Post-courtship, breeding birds have yellow green legs and dark yellow irises. The bill becomes yellow, although often retaining a bit of red to orange color at the bill base. After nest failure or break-down of a first pair bond, courting color is regained after a few days and the mate attracting process is repeated (M. Maddock pers. comm.).
Variation: Males are larger than females in most measurements (Mugica et al. 1987) and have more intense breeding colors. The two subspecies differ in their breeding colors and morphology. Ibis has rufous breeding color on it crown, chest and lower back. Coromanda has deep gold to dark cinnamon color more widespread, covering the entire head, neck and chest and most of the back. It also has a slightly thicker, longer bill and more extensive feathering on the tibia.
Juvenile: Juveniles are similar to nonbreeding adults. They may show a grey tinge to their plumage and have very dark to black legs. Maddock (1989, 1993) found that for coramanda, marked one-year-old birds returning to the nesting colony for the breeding season ranged in color from all-white, except for a buff patch on the crown, through pale patchy buff coloring to full orange adult plumage. McKilligan (1985) had proposed a two category color classification (pale and orange) but Maddock (1989) found that the color range could be classified in 4 categories: white 40%, pale (weak patchy buff) 29%, full pale (full coverage of pale buff) 9% and full color (the same strong orange coloration as 2 year and older adults) 22%. Birds in all categories were found to be nesting.
Chick: Hatchlings have white down, with tan to blue green skin. It has a yellow, slightly down curved bill. Legs are variable, tan, horn, yellow green or olive green, but are darker in front than behind. Iris is yellow. The down on the crown is bushy. The skin turns grey with age. The bill turns grey tipped then black by day 10, and diffuse yellow grey on fledging (Maddock 1989b, 1993).
They may a grey tinge to their plumage and black to very dark legs. Some birds assume adult breeding plumage at ten months and breed at one year, although others remain all white at one year (Maddock 1993).
Voice: The “Rick rack” call is a typical Cattle Egret call. The first syllable is louder and higher than the second. It is given throughout the year in many circumstances, used on alighting and in the nesting Greeting Ceremony. “Raa” call is the threat call, given in defending a perch or nest site. The “Ow-roo”call is a call used by males in the Stretch. The related “Rooo” call is sometimes given by the female in the stretch. The “Thonk” call is also a defensive call, but a soft muffled one given during courtship by the male to the approaching female at the nest site. The “Ruk, rok” call is a quieter, more hoarse, more muffled version of the Rick Rack call given during courtship. The “Kraah” call is the defence call, louder, loner and more aggressive than the Raa call, given while defending the nest or young. The “Kok” call is the alarm call, given singly or in a series rendered “kok, kok, kok, kok”, often in response to the aggressive chicks. The “Kaka” call is a soft chattering contact, reassurance call, rendered “ka, ka, ka, ka” used in several situations, by unmated males, a softer version by mated pairs, and a harsh version following the Rick Rack call in the Greeting Ceremony. Young beg with a repeated “Zit” call, rendered “zit zit zit”. Bill Snap is used in the Forward, which in the Cattle Egret is a variable advertising display. Wing flaps create a thudding sound during the Circle Flight.
Weights and measurements: Length: 46-56 cm. Weight: 270-510 g.
Field characters
The Cattle Egret is identified by its short stature (due to its relatively short upper legs), short, stout, yellow bill, relatively large head with a distinctive jowl, thick relatively short neck, dark legs and haunched perching posture, and in breeding by its red bill and legs and buff head, chest and back plumes. It flies with shallow rapid wing beats usually in flocks, generally low to the ground, often in formation, neck back and legs extending beyond the body. Upon landing it may spiral from on high, side slipping to a landing.
It is distinguished from the Great, Intermediate, Eastern Great egrets and white Reddish Egret by its smaller size, shorter and thicker bill, shorter and thicker neck, and during breeding by its buff plumes. It is distinguished from the Little Egret, Snowy Egret and immature Little Blue Heron by its short, thick yellow bill, shorter and thicker neck, shorter upper legs, haunched posture and stocky appearance, dark green to black legs or yellow in breeding (but not black or light green), and during breeding buff (not white) back and head feathers. It is distinguished from the Eastern Reef-Heron by its smaller size, yellow (not horn) bill, in breeding buff crown and back, flocking behavior and avoidance of marine habitats. In flight, it may be distinguished up close by its buff wash, but at a distance it looks white and its wide wings make it appear a larger bird than it is. It is distinguished from the Squacco Heron by its lighter back, yellow bill, thicker bill, white or buff (not streaked) head, and more vertical posture when feeding.
Systematics
The generic placement of the Cattle Egret has been uncertain, leading to its being assigned to the pond heron genus, Ardeola, and to its own genus, Bubulcus. The uncertainty was due to difficulty unraveling the evolutionary relationships disguised by its morphological adaptations to terrestrial foraging. Biochemical studies have revealed it to be an Ardea (Sheldon 1987).
The relationship of the two currently recognized subspecies deserves additional study as their disjunct range and distinctive plumage, and perhaps body proportions, suggests they may be separate species. The taxonomic identity of birds of the Seychelles, recognized as seychellara, also needs re-examination based on an adequate sample size. It is possible that this population represents a mixture of an older influx of the Asiatic coromanda, followed by a more recent influx of ibis.
Range and status
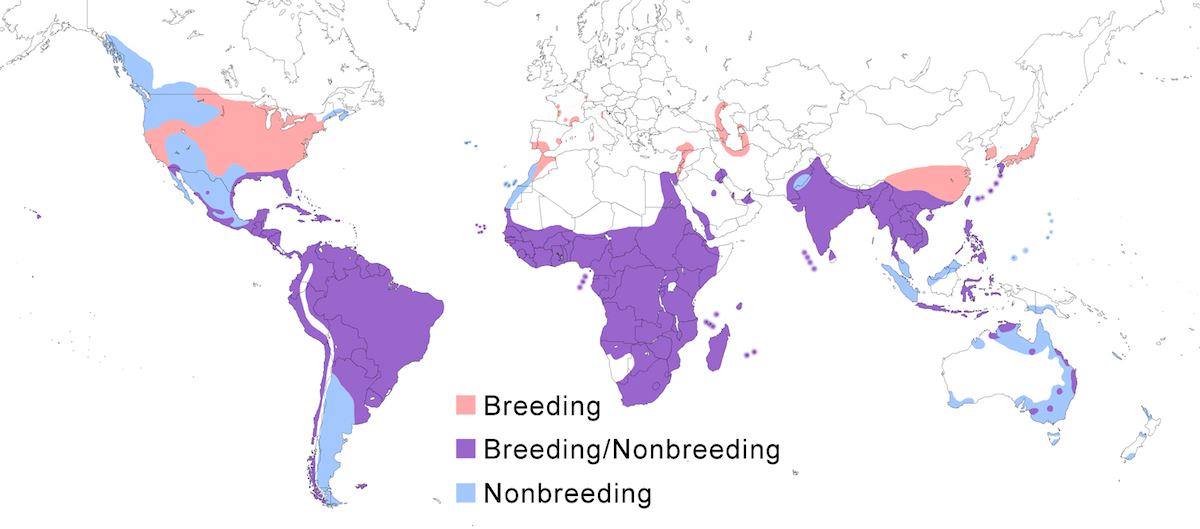
The species now nests across the mid latitudes to warm temperate zone in North America and South America, Europe, Africa, Asia, and Australia
Breeding range: Ibis breeds in north Africa (Egypt, Tunisia, Algeria, Morocco, Cape Verde Islands). In sub-Saharan Africa it breeds in Mauritania, Senegal, Gambia, Guinea-Bissau, Mali, Ghana, Niger, Nigeria, Chad, Central African Republic, Cameroon, São Tomé and Principe Islands, Congo Brazzaville, DR Congo, Sudan, Ethiopia, Eritrea, Djibouti, and Somalia. Also from Kenya, Uganda, Rwanda and Burundi south through all central and southern African countries except Zambia. In the western Indian Ocean, it breeds on the Comoros, Aldabra, Seychelles, Madagascar, and Mauritius. In Europe and the Middle East it breeds in Spain, Portugal, France (Marion et al. 1993, Marion 2000), Italy (mainland, Sardinia - Grussau 1997), Romania, Netherlands (Courtens 1996), Greece (Goutner et al. 1991), Turkey, Israel, Saudi Arabia, Yemen (Al Safadi and Kasparek 1995), south west Iran, Russia (Volga Delta), Azerbiajan, and Turkmenistan. In the New World it nests in Canada (Ontario, Saskatchewan), United States (breeding in all but 6 states), Mexico, West Indies, through Central America and South America except mountains to north west Chile (Antofagasta), Argentina (Santa Fe, Buenos Aires) (Azevedo 1997, Do Nascimento 1999), Hawaiian Islands (introduced, Paton et al. 1986, Ohashi and Kimizuka 1988), Galapagos Islands, and Falkland Islands.
The Asiatic race coromanda is separated from ibis by a broad gap across Iran. It breeds south of the Himalayas in Pakistan, India, Sri Lanka, Maldives, Nepal, Bangladesh, south east China (mainland, Hong Kong, Hainan, Taiwan), South Korea, south Japan (including Ryukyu and Bonin Islands), throughout southeast Asia except peninsular Malaysia – (Landsown pers. comm.) in Cambodia, Laos, Vietnam, Myanmar, Thailand (Round 1994), Nicobar and Andaman Islands, Indonesia (Java, Celebes, Moluccas, Sundas, Kalimatan, Sumatra), Australia (east Northern Territory and Kimberly Western Australia, east Queensland and inland western and coastal New South Wales.
Nonbreeding range: Cattle Egrets are partially migratory in Europe, with some birds remaining in southern France and southern Spain while others move to North Africa. Cattle Egrets occur in the nonbreeding season throughout sub-Saharan Africa. Eastern European birds winter in the Middle East (Iraq, Saudi Arabia, Iran), possibly extending into east and central Africa. Cattle Egrets breeding in Africa, shift in nonbreeding season in response to seasonal rainfall patterns, tending to move to the coast in the dry season (Baker and Baker in prep.).
In Asia, Cattle Egrets occur in nonbreeding season in Pakistan, India, south China, Indochina, Malaysia (peninsular, Sabah -Sheldon et al. 2001), Japan (Kyushu south), Philippines, Indonesia (Sumatra, Irian Jaya, Kalimatan), Papua- New Guinea, Micronesia (Carolines), Marinas (USA), New Caledonia (France). It has been recorded in New Guinea (Papua and Irian Jaya) for sixty years, but no nesting has yet been documented (Beehler et al. 1986).
In North America, Cattle Egrets winter in the United States south of 32° (with wintering concentrations in Florida, south California, Louisiana) into West Indies, Mexico, throughout the Central American range, into South America. In South America, they occur within the breeding range year round, but migrate south of their breeding range to Patagonia and the Falklands.
Some Australian birds winter outside the breeding range in south east and north west Australia, Victoria, Tasmania, New Zealand, Macquarie, Lord Howe, and Norfolk islands (Maddock and Bridgman 1992, Maddock 2000).
Migration: Many populations of Cattle Egrets are migratory and most show strong postbreeding dispersal, the two processes generally not being clearly separable. Cattle Egrets migrate in small flocks over extended periods mostly during high pressure conditions and avoiding rainy and foggy weather. They use flapping flight without soaring. Siegfried (1978) used an estimate of 50 km/h as the average speed of migratory flight to calculate a potential flight time of 20 hours for a flight range of 1,000 km. This estimate was supported by the Maddock and Bridgman study (1992), which would result in a flight to New Zealand taking 42 hours. A satellite study (Bridgman and Maddock 1997) recorded a flight overnight during migration at an average ground speed of 43 km/h over land. Optimum altitude of flight has been estimated at one kilometre (Maddock and Bridgman 1992).
Migration in this species has been best studied in Australia. Bridgman and Maddock (1994) found that eastern Australian autumn southward migrations after the breeding season were carried out in stages in northerly airflow along the western edge of a high, with passage of fronts and shifts to southerly winds halting movement. Movement to New Zealand was possible via south-westerly airflow both from NSW or from Tasmania. Return migration northwards up the coast in spring also took place in stages, but was more direct, with fewer staging points and occurred during southerly airflow. For return from New Zealand, the most likely scenario was found to be around the north of an advancing high in south-east, or easterly winds used as tailwinds near the margin of a high (Bridgman et al. 1998). At least some migration in Australia has been found to take place at night (Maddock 1990, Bridgman and Maddock 1994). Temperate birds tend to migrate before local winter, usually southward irrespective of location, whereas more tropical populations of Africa, Asia, and North America are more sedentary but have nomadic and dispersal tendencies.
European Cattle Egrets are partially migratory, birds move south and to the coasts. French Cattle Egrets tend to remain in south France. Birds from Portugal and Spain move south to the end of the peninsula, some crossing the Mediterranean to Morocco and north Africa. Egrets nesting in Turkey to Russia move south to the Middle East and also to east Africa (Ethiopia).
In Africa, birds breeding in north Africa appear to be mostly sedentary, while west African birds seem to be either migratory or nomadic, moving in response to rainfall. In southern Africa, birds move south to breed during the summer rains (November–February), moving north again to central Africa during the southern winter (April–August) (Turner pers. comm.).
In the New World, northern populations migrate, shifting to a southward movement after postbreeding dispersal, likely in response to the first frosts of the year (Byrd 1978, Telfair 1994). Migration is prolonged, September–November and later. They return in February–May. Western North American populations migrate to south California and west central Mexico. Central continent populations migrate through Texas, some remaining in coastal Texas, west to Baja California, central Pacific and Gulf coasts of Mexico, to Panama. Eastern Cattle Egrets move south to the Gulf Coast and to Florida, passing around the Gulf to join Central Flyway birds, wintering in Florida, passing through Florida to the Greater Antilles and north South America, or passing transGulf from Florida to Mexico, Central America to Colombia. The establishment of a strong migratory tradition in this invader is intriguing. It is possible that the West Indies-South America to North America route developed by following other migrating herons (Arndt 1988).
In Asia, northern coromanda are migratory, while southern populations appear to be more sedentary. As in West Africa, migrations in India correspond to the rain and dry season. Japanese egrets move to the Philippines. South China egrets move to Japan, Philippines, Indonesia (Borneo) and Micronesia (Carolines). Western egrets move to and through Malaysia (Malay Peninsula), Indonesia (Sumatra, Irian Jaya), Papua- New Guinea, Micronesia (Carolines), Marinas (USA), and New Caledonia (France).
In Australia, the Cattle Egret is partially migratory, but is likely better considered to be migratory over short distances (Baker 1978, Maddock 1990, McKilligan et al 1993, Maddock and Geering 1993, 1994). Migratory movements may take place in any compass direction but the majority of birds nesting in colonies in central and north coastal Australia move south and to a lesser degree north along the coast, although some have been recorded moving inland. Movements vary from short shifts to migrations of 2,000 km (Maddock 2000). Egrets move in stages to Victoria, Tasmania and New Zealand in April- May, returning to colonies in coastal eastern Australia in October–November. Project Egret Watch records show that Cattle Egrets tagged in the Macquarie Marshes in inland western NSW have been seen alive in Victoria in the south and in northern Queensland during winter (M. Maddock pers. comm.). It is possible but not proven that north Australian birds move northwards to New Guinea.
In both South America and Australia, southward migration to Patagonia and to Tasmania and New Zealand respectively results in birds moving from a warmer climate to a cooler one for the winter
Among all herons, the Cattle Egret one of the most highly accomplished in dispersal. It has occurred in many places around the world, crossing water gaps to islands, and being seen by ships at sea. Individual dispersal can cover long distances, 1,900-5,000 km in North America (Telfair 1983, 1994). It has been recorded in all Canadian provinces and all but two states in the United States (Telfair 1994). Off South America it occurs on Tierra del Fuego (Chebez and Gomez 1988) and also on South Shetland Islands (Mönke and Bick 1990) and Antarctica (Kaiser et al. 1988). In Europe, dispersal records include England, Ireland, Iceland, Belgium, Netherlands, Denmark, Sweden, Greece, Bulgaria, Rumania, Yugoslavia, Malta, In Africa, South African juveniles have been recovered in Zambia, Zimbabwe, Tanzania, Congo, Uganda, and Central African Republic. Highly nomadic west African birds have reached the Azores, Madeira, Canary, Ascension, St. Helena, Tristan da Cunha, and St. Peter and St. Paul Rocks. It is likely that one-way movements from Africa to South America continue by this route, propelled by trade winds (Orgeira 1996. In Australasia, it has occurred on Mariana, Micronesia (Schipper 1985), Guam, and the Aleutians (Alaska, United States - Gibson et al. 1988).
Status: This is a widespread and abundant species, perhaps the most abundant heron worldwide. Its range expansion during the 20th Century, throughout the Western Hemisphere has been sensational. Range change in the Cattle Egret is one of the more intriguing heron stories of the last century (Franchimont 1986a, Arendt 1988, Telfair 1994, Maddock and Geering 1994). Ibis occurred historically in tropical and subtropical Africa. But starting in the last century, it began to expand its range. It reached South America in 1877-92, south Europe also by late 19th century, nesting proven in 1944, South Africa in the 1920's, the Caribbean in 1933, the United States in 1941 nesting in 1953, Canada in 1952 nesting by 1962, Mexico in 1956 nesting by 1963, and Central America in 1954 nesting by 1958. It was introduced in Hawaii in 1949. Coromanda also expanded it range. I occurred in Australia in the late 1800’s but was first documented as nesting only in 1954. Once thought to be introduced, it in fact invaded Australia naturally (Maddock and Geering 1994, Maddock 2000). It occurred in New Guinea in 1941. From Australia, it reached New Zealand in 1963, although it is possible that it may have been as early as 1956 (Brown 1980). It has not been shown to nest there.
In North America it achieved a remarkable population expansion and is now the most abundant heron in North American, numbering over 400,000 birds in the east United States alone in 1970’s. Since the 1970’s the rate of increase has decreased and the population appears to have stabilized (Telfair 1994, Telfair et al. 2000).
In Europe it is now the second most abundant heron with a population of 80,000-100,000 pairs, about 66% of which are in Spain and 29% are in Portugal (Marion et al. 2000). Winter surveys found 150-160,000 individuals in the Iberian Peninsula (Sarasa et al. 1993). Although expanding slowly elsewhere in Europe, it is increasing in France reaching about 4,300 pairs (Marion et al. 2000). A sudden increase in nesting numbers and dispersion over most of France in 1992 corresponded to drought conditions in Spain (Marion et al. 1993), showing how dispersal events can trigger population expansion. Populations founded then persist (Marion 1997, Rimbert 1997). The nesting population on the Cape Verde Islands appears to have died out (Summers-Smith 1984). There are a few pairs to dozens of pairs in Italy, Russia, Turkey, Romania, Netherlands (since 1998) and Greece. It is increasing in Sardinia. .
It is common through most of Africa except in deserts and forests. Populations are increasing and large colonies are being found throughout the continent (Turner 2000), including 20,000 pairs in Morocco, 63,000-65,000 pairs in Mali (inner Niger Delta), 1,200 pairs in Kenya (Tana River), and 10,000 pairs in Tanzania (in the Wenbere Swamp alone) (Baker and Baker in prep.), 2,000 pairs in Zimbabwe, 1,700 pairs in South Africa (Hafner 2000, Turner 2000). It is expanding its range in Algeria to inland sites and in Egypt following a decline in 1970’s and 80’s (El Din 1992). In December 1995, a winter roost of 260,000 birds was counted in the Central Niger Delta in Mali. It is common throughout Madagascar, with one colony exceeding 1,000 pairs. In the Mid East, it is increasing in south west Iran (Perennou et al. 1994, Hafner 2000). In Azerbiajan, 10,000-15,000 pairs were found (Krivenko 1991).
In Asia, little information is available from the west. Further east, it is common and increasing in Pakistan (Sind, Punjab) (Roberts 1991). About 2,300 pairs were estimated recently in colonies in central China (Fasola et al. pers. comm.). It is the most abundant heron in India (Subramanya 1996), but it has declined in Japan where it was once abundant.
In Australasia, it is widespread and common in Australia wherever suitable pasture habitat with grazing animals is available. The population expanded rapidly in the east of the continent from first breeding recorded in 1954. By 1978-79, there were five nesting colonies with about 2,300 pairs in NSW (Morris 1979). By 1991, the number of coastal NSW colonies had expanded to 12, totaling more than 10,000 pairs (Baxter 1992). Project Egret Watch records suggest that there has been no further expansion since the 1989-90 breeding season (Maddock 1999). It is also common in New Zealand as a nonbreeding species, peaking at 2,200 birds in 1987 before declining.
Habitat
The habitat used by this species is one of its defining characteristics. Throughout its range, it forages in native grasslands and in pastures alongside hoofed livestock. In the area of origin of ibis, it forages in the open grass on the savannas and plains subject to annual inundation and slow drying, and associates with native grazing mammals (Siegfried 1978). Such rainfall dependent grassland has been simulated on a massive scale by man-made pastures managed for livestock through use of surface irrigation, ponds, tanks, wells and canals. Nearly world wide, during the 20th Century, as forest was cleared and improved to provide pasture for livestock, additional habitat was created for Cattle Egrets. It is primarily use of this habitat that allowed the egret’s range expansion and consequent population explosion. Changes in cattle ranching coinciding with population increases in the Cattle Egret are documented in Guyana, Florida, South Africa and Australia (Siegfried 1966b, Voisin 1991, Turner 2000, Maddock 2000).
Cattle Egrets do use aquatic habitats, and in fact do favor damp to recently flooded or poorly drained pastures over dry pastures and surface irrigated fields over dry fields (Mora 1992, Maddock 1995). They feed at the margins of many aquatic areas - riverbanks, stream edges, ponds, and shallow marshes - usually in association with other wading birds. They tend to avoid full marine habitats and deep forests.
Of critical importance is their use of artificial sites beyond pastureland. These include crop fields such as irrigated alfalfa fields, dumps, parks, athletic fields, golf courses, meadows, rice fields, lawns and road margins (Franchimont 1986b, Mora 1992). They will form large flocks to opportunistically follow behind ploughs and cultivators at work (M. Maddock pers. comm.). Rice fields are particularly important throughout its range including India, Sri Lanka, France, Spain and Italy. In France, Cattle Egrets selectively foraged in rice fields and other agricultural habitats, which afforded them a greater intake than natural habitats (Lombardini 2001).
They nest in colonies with other wading birds on a variety of substrates, marshes, reed beds, mangroves, dense thickets, bushes and trees - usually located on islands over or surrounded by water. Overall the Cattle Egret can be expected to occur where grasslands are present, where temperatures are not limiting to insect activity, where insect abundance is high, and where isolated nesting locations are available with wetlands nearby. It occurs from sea level to 1,500 m in India, and reaches 4080 m in Peru (Fjeldsa and Krabbe 1990).
Foraging
The foraging and food of the Cattle Egret has been exhaustively studied throughout its range (Heatwole 1965, Siegfried 1966a, c, 1969, 1971a, c, 1972a, 1973, Fogarty and Hetrick 1973, Thompson et al. 1982, Breden 1984, McKilligan 1984, Ruiz 1985, Newton 1986, Acosta et al. 1990, Metz et al. 1991, Yen 1991, Telfair 1994, Mathew et al. 1997, Kopij 1999, Lekuona and Artazcoz 2000, Lombardini et al. 2001).
The Cattle Egret specialises in Walking Slowly on land. It characteristically follows moving cattle, native large mammals, birds, or tractors capturing food that is made obvious by the movement of the beater. In Africa, its primary natural beater was probably the African Buffalo (Syncerus), but it also follows other grazing mammals, zebra, elephant, rhinoceros, giraffe, eland, waterbuck, or topi and other species such as ostrich. Elsewhere, it typically follows cattle, but also uses other species such as deer, capybara, camels, horses, feral water buffalo, sheep, goats, chickens, geese (Anseranas), and cranes. Cattle Egrets prefer animals that move forward at the most appropriate speed for their own Walking foraging. When following farm, mowing, or land moving equipment, it uses Leap Frog Feeding, in which birds land at the tractor, remain in place foraging, and then fly to the moving head of the flock again. It also uses this behavior without farm equipment, the flock serving as its own beater (Newton 1986). In commensal feeding, Cattle Egrets primarily hunt for small abundant insects and increase feeding effectiveness by obtaining more food with less effort, than do birds feeding alone (Heatwole 1965, Thompson et al. 1982, Scott 1984).
In commensal foraging, the behavior used is to Walk Slowly adjacent to the animal, usually toward its front quarter, observing prey made obvious by the beater’s movements. It walks with its head alternately withdrawn and then pulled forward with each step, a gait characteristic of the species. Cattle Egrets will typically dash a few steps after a prey item, Stabbing, Gleaning it off a plant, or Flycatching it in flight as it flees the egret or its beater. Cattle Egrets will also stop briefly, Stand and Head Sway, which allows better binocular discernment of stationary prey, or Neck Sways, which probably subtly encourages prey to move. Head Swaying seems to be associated with the hunting hard-to-catch prey. It will often ride on a large mammal’s back and Peck, Standing Flycatch, or Hop down to a prey item. In West Africa, Cattle Egrets have been observed Picking flies off Sitatunga antelopes (Tragelaphus), including also deep probing in the ears (oxpecker-like) for such insects (Turner pers. comm.).
Feeding commensally or otherwise, Cattle Egrets also use other supplementary behaviors when called for, including Walking Quickly, Running, Hopping, and Aerial Flycatching. Egrets feed without beaters, especially in situations where mammals are not available or when stalking high-energy vertebrate prey (Scott 1984). Typically the egret Walks Slowly in the grass or along wet margins. Neck Swaying is common during solo feeding, as is Head Swaying. Egrets are attracted to and follow fires, feeding on fleeing insects. They also will Stand, usually peering into water, and wait for a prey to come into view. It can feed arboreally, in trees and hedges where insects are concentrated and feeds on flies attracted to garbage or dead animals.
To catch their prey, Cattle Egrets typically Stab at prey. In addition to Stabbing at prey, they may also use Probing and Pecking at food items and have been observed and cases of Bill-vibrating have been reported.
Cattle Egrets are highly social in feeding. They are seldom totally alone and usually feed in flocks or loose aggregations of dozens to a thousand birds. Flock feeding is so core to the species’ behavior that it is likely that it provides some commensal advantage. Birds observe other birds foraging, thereby creating aggregations in places where prey is available (Metz et al. 1991). In flock feeding, egrets may be aggressive in defence of feeding sites or feeding areas, such as defending its place by the side of a commensal animal. They use Supplanting Run with a stiff legged stride to displace a competitor. Raa calls may be given. Runs may become a Supplanting Flight, with Raa and Rick rack calls by the alighting bird. Other threat and appeasement behaviors used are the Upright, Forward (in which the aggressor erects plumes, lifts wings, retracts head, rocks forward and stabs with bill snap), and Head Flick. The subordinate display is a Withdrawn Crouch.
It feeds during the day, most actively in the morning and afternoon. During midday and at other times when grazing stock rest to ruminate, foraging flocks often loaf with other birds in trees or on the ground near the resting herd. At night time it roosts with other species, sometimes in the thousands. The birds travel from the foraging grounds to the night roost site in late afternoon, often flying low along a watercourse. They form a pre-roost gathering at a nearby location to loaf, preen and drink (Blake 1969, Siegfried 1971b, Raine 1996, M. Maddock pers. comm.). In the last few minutes before dark, they fly to the roost trees or reeds. (M. Maddock pers. comm.). Individual birds tend to take up the same roost perch for the duration of the period of use of the roost (Siegfried 1971b, Maddock unpublished data). Along the migration routes and at wintering destinations large permanent night roost sites are used each year on both outward and return journeys. Smaller temporary roosts are also established at staging locations (M. Maddock pers. comm.).
DIET: This species has a diverse diet that varies according to habitat. However, the Cattle Egret is primarily an insectivore and is, moreover, a grasshopper specialist. Locust, grasshoppers, and crickets are the common element of its diet worldwide, and its specialized feeding behavior seems designed to find and catch orthopterans. Other insects are also eaten including flies (Tabanidae, Calliphoridae), beetles (adults and larvae), caterpillars, dragonflies, mayflies, and cicadas. Ticks are found in the diet but in small numbers; evidence for picking ticks off their commensals is slim. Ticks are probably found on vegetation. It certainly is not a “tick bird” in the sense of habitually gleaning ticks off cattle. Other prey include spiders, earthworms, mollusks, crayfish, frogs, tadpoles, lizards, snakes, fish, rats, and birds.
Vertebrates, especially frogs, are important during late nesting to provide the high energy packets and calcium needed for development. Chicks are selectively fed vertebrates, the proportion of which increases in the diet with chick age (Kopij 1999a, McConnell and McKilligan 1999). In Australia, advanced chicks were found to have been fed 16 species of reptiles and nine species of amphibians.
Breeding
The breeding biology and behavior of the Cattle Egret have been well studied (Blaker 1969b, Lancaster 1970, Siegfried 1972b, Hudson et al. 1974, Telfair 1983, 1994, Fujioka 1984, 1985a, b, Ploger and Mock 1986, Mock and Ploger 1987, McKilligan 1985, 1987, 1990b, 1996, 1997, Rodgers 1987, Arendt and Arendt 1988, Ranglack and Marion 1988, Voisin 1991, Ranglack et al. 1991, McKilligan et al. 1993, Wen and Sun 1993, Rao et al. 1996, Kopij 1999b, Si Bachi et al. 2000, Gassett et al. 2000).
The nesting season is variable, depending on food availability. In the temperate north, its nests in spring and summer, April–May in North America, April–July in Europe, June–August in north India. In the tropics, breeding generally takes place during or immediately following the rainy season, as grasslands begin to dry out. Nesting periods are mainly March–November in west, central and east Africa, September–March over much of southern Africa including Madagascar (Turner pers. comm.). Breeding season is November–February in south India, December–March in east Java, February–July in west Java, September–February in south east Australia.
It nests in reed beds, bushes, shrubs, or trees in protected locations, usually over or surrounded by water. Nesting colonies are found in islands in fresh, brackish or salt water, wood lots over dry land, and in swamps and marshes. In the New World, they are attracted to colonies established first by other herons and may depend on these early nesters (Belzer and Lombardi 1989). There is not a clear relationship between colony location and environmental conditions around the colony site, probably because most sites are chosen by aquatic herons. Conversely, Cattle Egret colonies appear to entice other herons to move more inland.
Cattle Egrets are highly colonial, breeding in mixed species colonies of a few hundred pairs to several thousand pairs including other herons, storks, ibises, darters, cormorants. They may be anywhere from the first to the last species to nest, depending upon geographical area. Their effect on other herons nesting with them has been a matter of some debate but evidence of meaningful impact is limited. The vast majority of early arrivals back at the breeding colony after migration are two-year old and older birds (Maddock and Geering 1994), with some one-year old birds not returning until December, two months after commencement of nesting (M. Maddock pers. comm.). Project egret watch records of tagged birds demonstrate faithfulness to natal breeding colony during a bird’s lifetime (up to 8 years, M. Maddock pers. comm.) with very few birds found to nest at a different colony (Maddock and Geering 1994). Faithfulness to very specific locations at wintering destinations (up to 5 years) has also been demonstrated (Maddock and Geering 1994).
The nest is made of reeds, twigs or branches; most are unlined. Characteristically Cattle Egrets often use leafy twigs. The nest is a shallow cup 40 cm wide (17-61 cm) and 12 cm deep (5.1-27.9 cm), with a pronounced cup. Nests are placed in reed beds, bushes, or low or tall trees up to 20 m high. Both sexes participate in the building; the male generally brings material, the female doing most of the constructing. Construction appears rather haphazard with many sticks not being successfully integrated into the structure. The nest construction takes 4-5 days, although the pair continues to add to its construction until egg hatching.
Small groups of males gather to display together, each male establishes its territory and becomes highly vocal and aggressive. It claims territory and begins displaying. Females are attracted to the displaying males spending time nearby, adopting a characteristic extended necked peering posture with crest partially erected. Territory claiming and defense from other males and initially by approaching females is by Standing, Twig Shaking, and Upright. The primary defensive behavior, and also a courtship display, is the Forward, in which the plumes are partially raised, bill is open, neck extended forward, and the male gives a Raa call, replaced by a Thonk call prior to pair formation. Forwards space out displaying birds. Supplanting Runs displace intruders. If aggressive displays are unheeded they become prelude to vicious fights made up of Bill Duels, Bill Jabbing, Face to Face Fighting, and Aerial Fighting.
Males display using several behaviors in addition to defensive ones. Head Shaking is accompanied by the Rick Rack call. Wing Spread is used after a Circle Flight, before a Forward, and while walking. This is a frequent display, in which the wings are spread downward with rocking movements as if balancing and all plumes partially or fully erect. The Snap is complete with bill snapping. The Stretch is not as obvious a display in Cattle Egrets as in other species. The bird stretches its head and neck vertically, legs partially flexed, body at about a 45° angle. The bird then retracts its neck, flexes legs, raises its scapular plumes, and gives the Ow-roo call. The “ow” is at the top of the stretch movement; the “roo” is on the downward movement. Display birds take Circle Flights making loud thudding sounds with their wings and also give Rick, rack calls. The Twig Shake in this species has more of an advertising function than is usual.
Females encroach on the male’s territory, usually from the rear being unable to withstand the male’s defensive behavior. Once they persist for a few hours, the bond forms quickly. After pairing, birds use several reinforcing displays. Bill Clappering and Back Biting are the primary reinforcing behaviors. Females give a Stretch using the Rooo call. Males Twig Shake. In the Greeting Ceremony, the back plumes are fully erected and the crest flattened. The male may present a twig. Pair formation lasts 3-4 days. Different mates are chosen each season, usually in a different tree or location within the colony and different mates are also selected for re-nesting after nest failure, which is a common occurrence (M. Maddock pers. comm.).
Copulation is preceded by a female Stretch, apparently an invitation, or by no obvious display. Unlike other herons, female crouches and gapes the bill upward during copulation, with the mounted male nibbling her crown feathers. Extra-pair copulation, wanted or unwanted, is common (Fujioka 1987, Fujioka and Yamagsihi 1981, McKilligan 1990b, M. Maddock pers. comm.).
The eggs are white with a pale green or blue tinge, broad oval and somewhat pointed, lighter than most other medium sized egrets. Sizes vary broadly from 40.4-52.1 x 29.8-36.5 mm. The clutch is usually 2-5 eggs, averaging 2.6 in Ghana and East Africa, 2.2 in Zimbabwe, 3.0 in South Africa and Madagascar (Kopij 1997), and 3.6 in Australia (McKilligan 1955). Eggs are laid at 2-day intervals. Cattle Egrets will renest after nest failure if not too late in the nesting season. Incubation begins with the first egg, and lasts about 24 days, ranging from 21 to 26 days. Both parents incubate, exchanging 1-4 times per day.
Eggs hatch asynchronously, 1 to 2 days apart. Hatching success varies from 14% in one study in south United States to 97% in north United States, in most areas about 50-90% (Ranglack et al. 1991, Kopij 1997, 1999b). Nestlings are altricial. Chicks soon become especially vocal and aggressive. Both parents share care of the chicks in about equal time proportions (M. Maddock pers. comm.). They are guarded until day 10, and shaded in the tropics. Both parents feed them. Chicks initially weakly grab at parent’s bill, becoming more aggressive with age. Being the larger, the first chicks usually are fed first, but this preference seems not mediated by the parents.
Maximum growth rates are at 9-12 daays, as endothermy is attained. They are fully feathered at 13-21 days. Young leave the nest and climb in nearby branches at two weeks. They fledge at 30 days, becoming fully independent in 15 more days. Legs change from dark green to black prior to fledging.
Success is usually fairly high; reproductive success was 94% in Texas (Telfair 1983), 37% in South Africa (Kopij 1997), although only 15% in Alabama (Dusi and Dusi 1970). In Australia, the mean number of chicks fledged by successful nests in an 11-year study was 2.42, with 41% of nests fledging 3 chicks and 6% fledging 4 or 5 chicks (M. Maddock pers. comm.). Rate of complete nest failure was high (41%), the majority of which were by nests in which one or both partners were one year old.
An important mortality factor is predation, by lizards (Varanus), crows (Corvus), ants (Solinopsis), owls (Bubo), hawks (Buteo, Parabuteo, Cicus, Falco), night herons, eagles (Aquila, Haliaeetus) raccoon (Procyon), and others. In Australia, unlike other herons, nesting success is not related to rainfall, except for the effects of severe drought (Maddock and Baxter 1991, Maddock and Geering 1994, M. Maddock pers. comm.). In India, drought does reduce nesting success (Gopakumar 1991). Strong winds, parasites, and human disturbance also cause mortality (McKilligan 1987, 1996, Kopij 1997).
The dominant cause of nestling mortality is starvation. Sibling aggression is frequent and severe in some areas, the extent of which is related to food supplies (Fujioka 1985a, b, Creighton and Schnell 1996). In tropical Africa, asynchronous hatching probably was related to unpredictable food supplies. In South Africa starvation of third and fourth chicks is inevitable. But in its expanded range, using artificial habitat, resources are in some cases abundant and nesting is limited by the ability of parents to deliver food. In New World studies, 98% of third chicks and 97% of fourth chicks survive to fledge (Telfair 1983), explaining its rapid population increase. In an 11 year study of breeding success in Australia (M. Maddock pers. comm.), very few chick losses of were recorded in 3, 4 or 5 chick nests. Forty two percent of successful nests fledging 3 chicks and 6% fledging 4 to 5.
Population dynamics
Nesting effort is related to rainfall patterns, leading to annual variation in productivity (McKiligan 2001). Birds can breed successfully at one year (Siegfried 1966a, Maddock 1993, 1989a). In the first year mortality is about 37-40%, which is a low figure for herons. In older birds, annual mortality is 20-28% (Siegfried 1970, McKilligan et al. 1993, Telfair 1994). Few banded birds are recovered older than 7-8 years (Telfair 1994), with the oldest bird seen alive in Australia being 11 years (M. Maddock pers. comm.). The life-span of adults in various populations is 9.1, 9.7, 11.1, and 13.7. These are much shorter than is presumed for other herons.
Predation and weather appear to be important mortality agents for Cattle Egrets. Birds are taken by Peregrines (Falco) on migration and exhibit avoidance behavior when peregrines are hunting (Telfair 1994). The cold winter in 1985 eliminated birds breeding in both the Atlantic and Mediterranean coasts of France, which were later recolonized by birds from Spain (Hafner et al. 1992, Marion et al. 1993).
Conservation
The Cattle Egret is a highly successful, widely dispersed, abundant species, probably the most abundant heron in the world. Its range expansion is continuing and population gains are being consolidated. While population conservation is not a global concern any species freshly invading new landscapes, especially one that characteristically uses human-made habitats. can come into conflict with people. The Cattle Egret usually is not persecuted on its feeding ground in that it is perceived to be beneficial or neutral to cattle activities. However, its tendency to develop large new colonies in and near towns and villages creates what is often perceived to be a public nuisance. To alleviate the noise, smell, health issues, water quality concerns, habitat degradation, and inconvenience of these colonies, local pressure arises to remove them or prevent their establishment, or even killing birds that appear to pose a health risk. Such actions have the unintended effects of shifting birds to other inconvenient sites and also can adversely impact other herons nesting with the Cattle Egrets, whose conservation status may be more of a concern (Dusi 1979, 1982, 1983, Fellows and Paton 1988). Control of nuisance colonies needs to be done within a regional planning context including environmental assessment, the use of non-lethal methods, the provision of alternative protected and managed sites, and public education. Beyond clearly nuisance colonies, nesting sites and their associated feeding situations should be protected and managed (Fernandez-Cruz et al. 1993). Cattle Egrets can adversely affect the trees and bushes used for nesting, leading to the abandonment of the colony site if it is not managed. Colony sites, and potential colony sites, need to be conserved on a regional basis. There also is a need for attention to the status of foraging habitat along migration routes. a species that forages in man-managed environne it could be expected that pesticides and other taminants would be a conservation issue, but despite many studies none yet indicate severe adverse effects (Heinz et al. 1985, Telfair 1994). A likely difference between contaminant burden in Cattle Egrets and other herons is that a insect diet has less potential for bioaccumulation than does a fish diet. Instances of high contaminant burden may reflect local practices, on the breeding or wintering grounds, and so can serve as indicators of environmental conditions (Burger et al. 1992).
Research needs
The Cattle Egret is one of the best-studied herons. Research opportunities lie at two scales, the population and the individual. The demography and population biology, such as nesting success, role of brood reduction, and perhaps survival patterns, appear to differ between its historic African range and its newly invaded range. Explicit study comparing the demography of the species in the two areas could unravel fundamental questions of demographic flexibility. There also appear to be fundamental differences in demography between the Cattle Egret and the more long-lived species. Cattle Egrets appear to be rather independent as individuals. Dispersal and fidelity, seem to be a matter of individual discretion. Movement, physiological, and genetic studies at population and individual levels could reveal much about such individual decisions. There is more to be understood about the relationship of the two subspecies of Cattle Egrets.
Overview
The Cattle Egret is an odd heron, being fundamentally a terrestrial insectivore. Although superficially homogeneous, the heron family exhibits a graded series of morphological and behavioural traits. The Cattle Egret is at one end—that of terrestrial adaptation. As a terrestrial walker, it has relatively short tarsus and small feet. It has a short bill, opposite to the long-billed fish specialists (Boev 1989), better for catching small arboreal prey. The bill is also relatively thick to handle hard-shelled prey. Its slow walking feeding is aimed at carefully examining grass and ground for motionless hidden prey, inspiring prey to move, and capturing it before it flies or hops away. Its association with mammals clearly increases its feeding effectiveness. So does its flocking. Although there is no evidence of persistence of membership in any group of Cattle Egrets, flocks develop a functional structure, moving in unison and in fact serving as their own beater.
Its preferred habitat is not grassland per se, but grassland that is damp or has been seasonally flooded. Likely this is due to such land being more productive of insects than dry grassland. In Africa, wet and dry periods are highly seasonal, and variable in extent from year to year. As a result Cattle Egrets need to be able to shift their populations in order to find and exploit the variable supply of damp or drying grasslands.
The species has a strong post-breeding dispersal tendency. It is predisposed to take advantage of human-caused habitat alteration, creating improved pasture, complete with buffalo-surrogates in the form of domestic cattle. Its dispersal skills brought it to new lands in sufficient numbers to colonize and to take advantage of such developing agricultural practices.
The importance of an insect diet to such a large bird is problematical. Herons, no matter how successfully redesigned, seem not to be optimal insectivores. That Cattle Egrets can specialize on insects is a wonder, especially when it is calculated that an egret needs to consume 1,728 grasshoppers in 12 hours to feed itself and its young, or a grasshopper every 25 seconds (Telfair 1994). This feeding productivity seems unlikely over the long run. In its historic areas in Africa, brood reduction is the rule. Nesting success there depends not on the egret’s primary insect prey but on its access to aquatic vertebrates, particularly frogs. So it appears that the Cattle Egret has limits to how far it can stray from its water-dependent ancestry. Brood limitation, while typical of ibis’s native situation, seems not the rule in North America. Released from its co-adaptive constraints and nourished by irrigated improved pasture land, the Cattle Egret’s enhanced reproductive potential enabled it to explode across the American landscape.
Cattle Egrets need to learn to feed. Juveniles feed in shorter grass, take similar number of steps, make fewer capture attempts, obtain half as much food items per time, have to feed longer, and also feed more alone (Burger and Gochfeld 1989). Some of these results are intriguing. Among what a Cattle Egret needs to know is how to feed in long rather than short grass, how to be efficient in capture, and how to take advantage of flock behaviour. Apparently it learns these things quickly in that it can breed before its first birthday, although not as successfully as adults.
High dispersal tendency, ability to breed in year one, high nesting success, potentially low chick mortality, low first year mortality but relatively short life span suggest that the Cattle Egret is indeed the “r” strategist among herons, a species well adapted to exploiting changing environments through population increases.
The Cattle Egret is the heron success story of the twentieth century. It has expanded across the world, responding to human habitat change and is becoming an integral part of the human landscape nearly worldwide.