Eurasian Bittern
Botaurus stellaris (Linnaeus)
Ardea stellaris Linnaeus, 1758. Syst. Nat. ed. 10, p.114: Europe; restricted type locality, Sweden.
Subspecies: Botaurus stellaris capensis (Schlegel), 1863: South Africa.
Other names: The Bittern, Great Bittern, Common Bittern, European Bittern, African Bittern, Boomer in English; Avetoro Común in Spanish; Butor étoilé in French; Rohrdommel in German; Boerdomp in Dutch; Built in Russian; Rördrom in Swedish; Sankano-goi in Japanese; Da mayan in Chinese.
Description
The Eurasian Bittern is a stocky, thick necked, medium sized, mottled, black crowned, golden brown heron.
Adult: The Eurasian Bittern is the largest of the large bitterns. It has a black cap. The long bill is yellow with the top being brown and becoming almost black at the tip. The iris is yellow and a line above the eye is pale buff. The loral area is green, extending on to the lower bill. The sides of the head are uniform buff brown. The neck is buff brown narrowly barred brown. The chin and throat are cream white with rufous brown median streak. A weak black to dark brown moustache extends from the bill gape onto the neck. The hind neck and back are golden-brown, consisting of black and buff mottling and barring. Two “shoulder plumes” consist of elongated feathers with a brown centre and large white border, usually concealed by the folded wings. The upper wings are pale rufous buff, darker on the leading edge, and blotched with black. The flight feathers are pale red brown to black, with black spots. The foreneck and breast are yellow buff with longitudinal brown streaks and finely spotted black. The streaks are widest on the chest and narrowing on belly. The feathers of the foreneck are thick and elongated into a bib. The belly is yellow buff. The under wing is pale buff with grey mottling. Leg and feet are pale green. The soles of the feet and back of lower legs are yellow. In breeding the loral area is light blue in the male and light brown in the female. It is likely that reports of orange red irises are indicative of a color change in courtship (Wood 1986).
Variation: Males are significantly larger than females. The females may tend to be less well marked than the males. There is much individual variation in the dark plumage markings, some individuals appearing quite pale. There also is geographical variation. Birds from east Asia tend to be more boldly barred, and were formerly considered a subspecies. The disjunct birds of southern Africa, capensis, are slightly smaller and darker, with more narrow and irregular barring on the flight feathers than stellaris.
Juvenile: Juveniles are very similar to adults. They are paler with browner stripes, have a browner crown and less distinct brown, not black, moustache streak.
Chick: The chick has cinnamon tawny to rufous brown down on the upper parts. Chin and throat are white. The under parts are rufous buff. Bill and leg are blue green.
Voice: The “Boom” call consists of two to four deep, resonant booms repeated at 1-2 sec intervals, preceded by a few short grunts or pumps and sometimes accompanied by Bill Clappering. The sequence of pumps and booms in the Boom Call is variable among individuals and often among the calls of an individual (McGregor and Byle 1992, Gilbert et al. 1994, Puglisi et al. 1997). The call has been rendered as “up, up, up, rumb”, “umb, uh, uh, uh, ub”, “up, up, up, rumb”, and “hu, hu, umb, ub”. The Boom Call is used during the breeding season for advertisement and territorial defense (Puglisi et al. 1997) and is audible for up to 5 km. The female in answering the Boom Call uses the “Wumph” call.
The “Kau” call is a flight call, contact call and disturbance call; perhaps the multiple use of a call is not unexpected for a bird from dense habitat. It has been rendered as “kau” “aargh” “aark, aark” and “awk, awk”. The “Kro” call is a disturbance or warning call, rendered as a rapid, short “ko, ko, ko”, and “kro, kro, kro”. The “Kra” call is a threat or warning call. Young beg with a crackling “rah, rah, rah.”
Weights and measurements: Length: 70-80 cm. Weight: females 867-1,150 g; males 966-1,940 g.
Field characters
The Eurasian Bittern is identified by its stocky build, thick neck, medium size, golden brown mottled plumage, black crown and dark moustache. If disturbed it flies up with neck extended and legs dangling, and quickly drops back into the reeds. In long flight it has steady slow wing-beats, on broad rounded wings. Described as owl-like, it flies low over the marsh, with head retracted and legs trailing.
It is distinguished from the immature Black Crowned Night-Heron by being larger with a heavier, stockier build and less hunched appearance, having back and upper wings mottled with black (not densely spotted white), black crown, and moustache. It does not flock or perch in trees. It is distinguished from the North American Bittern by being larger, with a black (not rusty brown) cap, indistinct black (rather than prominent large) moustache stripe on the head, more boldly marked plumage, broader wings without dark flight feathers (Lansdown 2000).
Systematics
The Eurasian Bittern is one of the four large Botaurus bitterns, which all have streaked brown plumage, scutellate tarsi, 10 tail feathers, and a booming call. It is most closely related to the Australasian Bittern (Payne and Risely 1976, Sibley and Monroe 1990).
Range and status
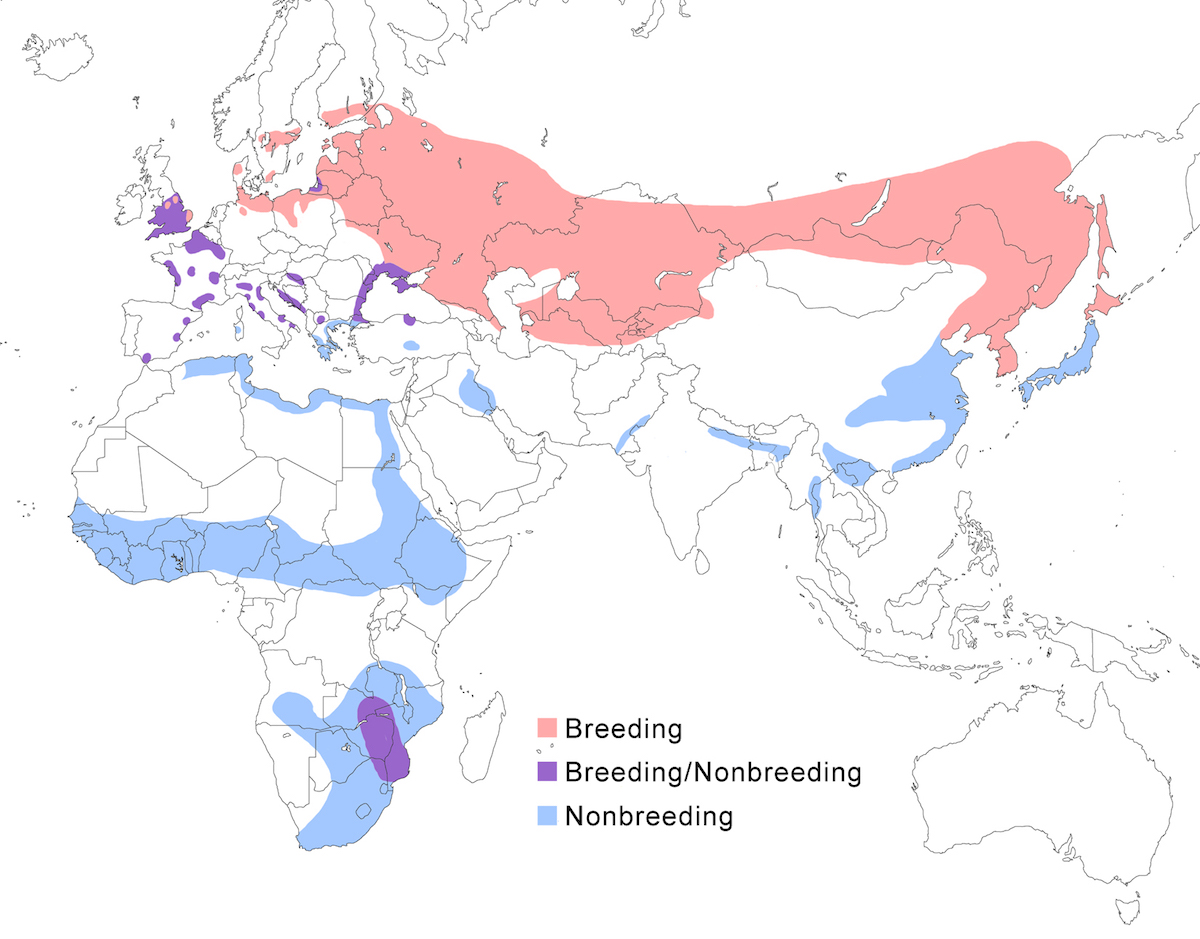
The Eurasian Bittern is a bird of the Old World temperate and tropical zones of Europe, Asia and Africa.
Breeding range: It breeds from England, Wales, Sweden, Finland, Germany, Lithuania (Matiukas 1990), Italy, Belarus, European Russia), south through Europe, north Africa (north Morocco, Algeria) Albania, Bulgaria, Turkey (Kasparek 1986), north Iran, Kazakstan, Azerbaijan, Turkmenistan, Uzbekistan, Tajikistan, Kirghizstan, Afghanistan, Mongolia, north China (Sinkiang to Heilongjiang), Russia (Siberia to 64° N, Yakutia, Amurland, Ussuriland, Sakhalin Island), North Korea, South Korea, Japan (Hokkaido). Capensis is presently known to nest only in Natal and Transvaal in South Africa and probably Zambia.
Nonbreeding range: Stellaris winters from central Europe, east Europe to Ukraine,
east Saudi Arabia, Iraq, Iran, Turkmenistan, Pakistan, north India, Nepal, Bangladesh, south east China (including Hong Kong – Leader 1999), Korea, Japan (Honshu), Myanmar, Cambodia, Vietnam, south Thailand, north Malaysia (peninsular), also north Africa in Libya, Egypt, sub-Saharan Africa in Mauritania, Senegal, Ghana, Gambia, Gabon, Nigeria, Cameroon (Robertson 1993), Kenya, north Zaire, Sudan, Ethiopia, Eritrea.
The known breeding sites for capensis are few. However it occurs over much wide range in central and south Africa. Until proven otherwise, this is considered a nonbreeding range. They occur in central Angola, Namibia (Hines 1985-7), north Zambia, Zimbabwe, south Tanzania, Malawi (Johnston Stewart 1989), central Mozambique, northwest Botswana, north Seychelles, South Africa (Vernon 1993).
Migration: The north Eurasian populations are partially migratory. Some birds remain in breeding areas where water does not freeze, or stay further north in mild winters (Bibby 1981, Andersen 1989, Nankinov 1991, Kolland 1996, Mundt 1996, Gauggel 1999, Bernasconi et al. 1999). Some populations, such as England and Netherlands, are nearly residential.
Both Fall and Spring migration are primarily at night (Puglisi and Baldaccini 2000). Birds move singly or in small flocks. Southward migration is variable, seriously getting underway after the first hard frost, generally September to as late as December. West European birds move south through France and Spain; others move through Greece and Italy (Voisin 1991). They cross the Sahara where they occur in oases. Return migration is February–April. The origin of birds wintering in Iran and Iraq is not known, probably Russia (Voisin 1991). Asian populations move south to the northern half of the Indian subcontinent, Myanmar and throughout central China to the Chinese coast. Migration here is little understood (Leader 1999).
The African race capensis is mainly sedentary. Some movements take place, mainly to the southwest, and these are governed principally by rainfall.
Post breeding dispersal is extensive, especially of immature birds. Dispersal records include Iceland, Faeroes, Madeira, Azores, Canary Islands, Norway (Jebekk 1996), Syria, Jordan, north Russia (Pleshak 1999), south India, Myanmar, Sri Lanka (Gunawardene and Wijesinghe 1986), Ryukyu Islands, Taiwan, Malaysia (peninsular), Philippines (Luzon), Brunei (Mann 1987).
Status: Formerly widespread and abundant, the Eurasian Bittern has suffered a steady decline in numbers in Europe, since the 19th century, notably from 1970-1990 For example, it was extirpated from England in 1868. It began a comeback through Europe in early 1900's, returning to England in 1911. It increased population and range into the 1960’s and then began a second decline. At present, the bittern remains widespread in Europe, but with relatively few remaining in the west. Declines in some areas have been rapid and alarming, such as Spain but is reversed at other sites (Duhautois 1984, Smith and Tyler 1993, Puglisi et al. 1995a, Vicens 1997, Kayser et al. 1998, Marion et al. 2000).
The total European population is 20,000-44,000 pairs (Marion et al. 2000). Russia supports 63% of the European population, 10,000-30,000 pairs, but its status there is little known. Ukraine, Poland, Belarus, and Romania account for 24% of the European population. It is locally abundant in Turkey (Kazilirmak Delta) and Romania (Danube Delta) (Hafner 2000). In North Africa, it is very rare, occurring only in a few pockets of fresh water. In Africa records of capensis are sparse and has clearly has undergone a considerable decline, especially in recent years. It is known to breed in South Africa and is rare in Zambia. It is common in Iran but considered to be uncommon through its breeding and nonbreeding range in Asia.
Habitat
The Eurasian Bittern is a bird of densely vegetated wetlands with extensive beds of tall grass or sedge. Usually these marshes are characterized by being shallow, fresh or brackish, with stable water level, low acidity, low elevation (less than 200 m, and reed stands of varying ages interspersed with open water. Marshes dominated by cane, Phragmites are favoured in Europe. It also uses Scirpus marshes, papyrus swamp, brackish marshes, and coastal dune wetlands. Rice fields may be serving as a potentially important habitat (Alessandria et al. 1992). Outside the nesting season, its requirements are less restrictive. Beyond the reed bed, it uses more varied and open aquatic habitats such as small ponds, gravel pits, wet grassy meadows, ditches, fish ponds, water cress beds, other floating leafed plant beds, and sewage lagoons (le Mair et al. 1995). During winter, it tends to come out of the reeds to find open or running water in which to feed. In very cold weather the species may occur nearly anywhere there is open water.
Foraging
The Eurasian Bittern is a solitary feeder. It fiercely defends its feeding and nesting area during breeding, but may occur in larger loose groups outside of nesting. It is mainly crepuscular, it also hunts during the day and at night, within and at the edges or reed beds. The main method of hunting is Walking slowly, with great stealth. It walks in an extreme Crouched posture, the bill pointed forward, the feet lifted high with each step. It also feeds by Standing, often as an intermission in Walking. It also Walks Quickly, especially across open spaces and also Swims across open water between patches of cover (Lindblad 1995). It has been observed to Run across open habitat picking up prey items. It Foot Stirs to inspire fish into movement.
The bittern tends to feed at the edge between emergent reeds and open water, such as a pool, channel, or ditch. They tend to avoid unflooded ground and walks by climbing over the emergent stems, grasping them with long toes. They also climb up the stems for observations, and to sunbathe. They avoid shrub, which has consequences when marsh succession occurs.
Large areas are covered in the search for food. It captures prey by stabbing using its entire body. Fish are shaken, beaten and stabbed before consumption. It often uses the concealing Bittern Posture, in response to disturbance but also for surveillance as it can peer directly forward when its bill is raised; it has been observed to hold this posture for hours. The bittern generally is found on the ground or in herbaceous growth, but on occasion uses trees (Lunn 1992).
A wide variety of foods is taken, varying with locality and season. Fish, amphibians and insects usually dominate the diet. Fish taken are diverse depending on availability. Species include Esox, Eupomotis, Cyprinus, Salmo, Perca, Thymallus, Tinca, Anguilla, Cottus, Leuciscus, and Rutilus. The diet also includes frogs, tadpoles and salamanders (Rana, Triton, Bombina, Leuciscus), worms (Lumbricus), crustaceans, insects (water beetles, water bugs, grasshoppers, dragonflies, leaf miners), small mammals (Avicola, Mus), birds (Anas) (Loison 1991), snakes (Natrix). In some areas eels, frogs or tadpoles dominate the diet. The diet is particularly challenging for birds that remain north in the nonbreeding season, where they use atypical habitat and prey (Windisch 1999)
Breeding
The Eurasian Bittern nests in March–July in Europe, sometimes earlier; September–January in South Africa. Generally nesting is in the rains in the tropics, but there is little information. During the breeding season, the bittern is nearly restricted to dense reed beds. The ideal habitat is restrictive: dense plants, of both old and young stems, stably flooded, shallow, with clearings or channels. They may use either fresh or brackish water, but not saline. In addition to Phragmites, plants used include Scirpus, rice, Papyrus, Bolboschoenus, Cladium, Juncus.
Breeding is essentially solitary. Density varies from 2/100 ha to 100/100 ha, depending on the habitat quality. However under suitable habitat conditions, nests may be rather close together, as many as 10 were counted in 0.5 ha (200/100 ha). This is highly unusual, but nests are also close in situations where there are more than a single female associated with a male. The nest is a pad of matted reeds and other marsh vegetation, lined with finer material. It appears carelessly put together and flattened by use. Nests are 30-40 cm wide and 10-15 cm high, placed on and within the reeds at water level. It is built by the female alone. The bittern’s movements soon create radiating tracks through the nearby marsh. Material is added through the nesting period.
In Spring, the male claims its territory and advertises by giving is Boom Call (Fontanelli et al. 1995). Nonmigrating birds begin to call in late winter. The male will occupy and defend its territory through July. The Boom Call is produced with the bill closed and pointing downwards and neck feathers fluffed out. The Boom Call is most given at dawn and dusk, but also throughout all hours of the day and night (Puglisi et al. 1997).
Males defend their home range territories (Puglisi et al. 1999). They are extremely aggressive in its defence. Supplanting Flights occur, as does Aerial Fighting, which may end in the death of one of the bitterns. Forward displays are used to defend sites. The bird has its legs bent, neck bent back, bill open, wings bent forward, crest, neck feathers, scapulars, and shoulder tufts erected (Gorman 1995). Attacks are made by a strong beak jab, perhaps jumping forward. Chest pecking may be a ritualized outcome of low intensity attacks. Subservient birds respond with a Withdrawn Crouch. However, bitterns sometimes kill other bitterns. The bittern also defends against other species, particularly the Marsh Harrier (Circus) and Carrion Crows (Corvus), and is known to loose prey to pirating birds, such as Grey Herons (Kington 1991). Interactions between pairing individuals are seldom described. In close proximity, the shoulder plumes are displayed (Grull et al. 1988).
In addition to the Boom Call, displays include individuals or several birds indulging in extensive Circle Flights over the reed beds. The flying is erratic, at heights of up to 60 m and for as long as ten minutes, ranging over large areas of the habitat. When flying together the bitterns circle around each other or shoot upwards and downwards again.
This is the heron species in which true polygamous mating has been best shown. It is not unusual, and the male may have up to five mates. The eggs are olive brown, occasionally with some fine spotting at the large end. They average 53 x 39 mm. The normal clutch is 4-5 eggs; range is 3-7. Eggs are laid at 2-3 day intervals. The species has long been known to be single brooded, however double brooding has recently been documented (Mallord et al. 2000). Only the female incubates. Incubation begins with the first egg and lasts 25-26 days. The male continues to advertise and defend the nesting territory through several months, through June–July in Europe.
Eggs hatch very asynchronously. In a large clutch the last egg may hatch 12 or 13 days after the first, and these last eggs often fail to hatch. Young are semialtricial and nidicolous. They are cared for by the female. Polygamous males play no part in the rearing of the young. They are driven away by the female should they approach the nest. In monogamous pairs, the male may bring food near to the nest for the female to use. Vary rarely, the male joins the female on the feeding grounds. Food is regurgitated into the nest. Large prey not consumed by the young is often re-consumed by the parent. After 15-20 d, the young are fed off the nest site.
The young soon become very active, exhibiting various self-defence attitudes and actions. They clamber from the nest and through the reeds after the first week. They fledge at 50-55 days. Breeding success in one study in Germany was 56% reared to 14 days.
Population dynamics
Bitterns first breed as early as one year. Populations in which some birds remain north in winter can suffer significant mortality in hard winters, up to 40-80% (Marion et al. 2000). After a winter kill in the early 1960's, nesting sites were never reoccupied. In Sweden the population declined from 450 to 270-300 pairs after a hard winter and the populations needed 3-7 years to recover (Marion et al. 2000). Normal juvenile or adult mortality, or the role of migration is not known. Longevity is 9 years.
Conservation
The population in Europe has been in decline for many years. An original contributing factor was habitat alteration, mainly through drainage and human persecution. In some countries protective measures brought back the population by the middle of the last century, but in the past 30 years the decline has resumed. Although factors are unclear, it is likely that core to the decline is the continued loss of Phragmites marshes. Conservation activities in Europe need to concentrate on these habitats. In evaluating the decline of European bitterns, it is important to consider that similar declines are occurring in other large bitterns. Whereas habitat alteration is clearly involved in some areas, such in west Australia, causes remain puzzling in other areas such as New Zealand, and large-scale climatic events appear to be implicated elsewhere, such as in North America. The long-term and short-term impact of winter mortality on populations is clear (Bibby 1981). Information on the trend of the Eurasian Bittern in east Europe and Asia is important to begin distinguishing among causal possibilities.
The HSG considers the European and North African population to be Regionally Vulnerable (Hafner et al. 2000). Habitat loss, management practices that fail to maintain the habitat characteristics needed by bitterns, disturbance by humans, and pollution have been implicated in the declining status of the bittern in Europe (Marion et al. 2000). Human disturbance is pervasive in Europe. Cane cutting, recreation, motor vehicles, hunters, and boats all disturb nesting bitterns (Day and Wilson 1978, Newton et al. 1994, Tyler et al. 1998, Whiteside 1989, Piskorski 1999). Protection and management of important bittern habitats are needed across its European range. Fortunately research is demonstrating appropriate approaches (Bibby and Lunn 1982, Tyler 1994, Hobbs 1995, Welch 1995, Vicens 1997, Puglisi et al. 1995b, Dvorak et al. 1997, Tyler et al. 1998, Soto-Largo et al. 1998, Schlumprecht 1999, Smith et al. 2000). Reed cutting, for example, if properly managed, can provide multiage stands desirable to the bittern, but if badly managed—such as permitting large-scale cutting in late winter-habitat quality is destroyed. Regional management schemes, monitoring, education, and local action are needed, perhaps linked to conservation of the Purple Heron in these same habitats (Hafner et al. 2000). Wintering birds remain common in Iraq and Iran. There drainage of the Mesopotamian marches is a threat to their welfare and that of other water birds that use this critical area. Information on wintering status in Africa is nearly non-existent. The southern Africa population is even more at threat, given its rapid decline over the past several decades. Conservation action in its remaining known locations in South Africa and Zambia is essential.
Research needs
There have been important advances in our understanding of the biology and conservation needs of this species. Studies should continue on the breeding, habitat needs, and demography of this species especially outside western Europe, where most information is from. Surveys are needed to determine the status and distribution of nesting of Eurasian Bitterns in southern Africa and wintering birds in northern Africa. The status and trends of the species in Russia need to be understood, not only to develop appropriate conservation strategies but also to compare with the situation in west Europe.
Overview
The Eurasian Bittern is a skulking species seldom seen far from its reed-bed habitat. It spends most of its time Walking very slowly, but covering large distances around its feeding territory. It catches what it can, with considerable specialization in different locations. The Eurasian Bittern has polygamous tendencies and exhibits lack of a parental role by the male. This means that the feeding of the chicks is left mostly to the female, the male’s role being to defend the territory and protect food supplies for the female. The bittern has the largest span for asynchronous hatching among herons, the span of ages within the brood perhaps serving to separate periods of high energy demand among the siblings. Much remains to be understood about the biology of polygamy in bitterns.