Great Egret
Ardea alba (Linnaeus)
Ardea alba Linnaeus, 1758. Syst. Nat. 10(1), p. 144: Europe.
Subspecies: Ardea alba alba Linnaeus. Ardea alba egretta (Gmelin), 1789: in insula S Dominici, insulis Falkland et America australi ad Louisianum usque; Ardea alba melanorhynchos (Wagler), 1827: Senegambia.
Other names: Great White Egret, Common Egret, American Egret, Great White Heron in English; Garceta Grande, Garzón Blanco, Garceta Real, Garza Real, Graza grande, Garza Blanca in Spanish; Garça branca-grande in Portuguese; Grande aigrette in French; Silberreiher in German, Grote zilverreiger in Dutch; Airone bianco maggiore in Italian; Большая белая цапля in Russian; Da bailu in Chinese.
Description
The Great Egret is a large, slender, white heron, with long neck, dark legs and long back plumes when breeding.
Adult: The Great Egret is an all white bird. The feathers of crown are slightly elongated but do not form a crest. The bill is yellow or yellow with black tip. Irises are yellow. Lores are green yellow to yellow. A black line of bare skin extends from the edge of the gape to beyond the eye. The head is without crown plumes. The legs are variably dark.
Breeding season soft part coloration varies geographically. The back plumes reach full development during courtship when as many as 50 plumes, up to 50 cm long, extend as much as 10 cm past the tail. These aigrettes have stiff quills and are able to be fanned in courtship. Feathers of lower neck and chest are thick and only slightly elongated and frayed but not fully lanceolate.
Variation: Sexes are alike in plumage, but males are larger than females. The races differ in soft part coloration, particularly during breeding.
Egretta has a dull, mottled, yellow bill, often tipped in black in nonbreeding season. Legs and feet are black. In courtship, the bill turns bright orange, then fading back to yellow. The iris has an outer red ring. The lores are a bright green.
Alba is the larger race. In nonbreeding, bill is yellow. During courtship bill is red with dark tip, turning to black with yellow base with red streaking. In breeding, lores turn bright green. In nonbreeding, leg color is variable upper legs are generally black with green or yellow tinge. In courtship, upper legs, and sometimes stripes on the lower leg, are pink. Post courtship during the remainder of nesting, the upper leg turns yellow and lower leg dark.
In melanorhynchos, the nonbreeding bill is yellow, lores yellow, and the legs and feet are black. During courtship the bill turns black and the irises turn red. The lores turn bright blue green. After pairing, the irises become yellow, the lores pale green then yellow green, and bill becomes yellow starting at the base leading to a bicolor condition usually with the tip remaining black.
Juvenile: Juveniles are similar to adults, but lack ornamental plumes. They have yellow bills with a blackish tip.
Chick: Nestlings are covered in white down, with elongated down on the head forming crest. The upper bill is grey with a black tip, the lower bill is yellow grey, both turning yellow. The iris is straw to green. The lores are pale blue grey becoming yellow. The throat is pink. The legs are pink becoming grey green. In melanorhynchos the skin is green becoming yellow.
Voice: A low pitched, deep, “Kraak” call is the flight, disturbance, and threat call. The high pitched “Eeeiee” call, rendered “eeeee, i, eeee”, also is a threat call. The “Cuk” call, a rapid “ cuk, cuk, cuk”, is also a disturbance call. The characteristic advertising call is the soft “Frawnk” call. The “Rha” call is a courtship call. In Africa a “Cra” call, rendered “craa, craa, craa” has been reported as the nest greeting. A “Rrrrooo” call, used in the Greeting Ceremony call, is also called the “Laughing Call”. Young beg with a “Ket” call, rendered “ket, ket, ket, ket”. The literature on vocalizations is not clear, suggesting additional geographic or racial differences likely exist.
Weights and measurements: Length: 85-102 cm. Weight: 930-1,700 g.
Field characters
The Great Egret can be confused with the other white herons. It is identified by its size relative to the other white herons, its back plumes, dense breast plumes, lack of crest and head plumes, yellow and yellow black bill, the extension of a dark line from the gape to beyond the eye, and dark legs. It has long legs and neck relative to the size of the body (Boev 1988a, 1989); the strongly kinked neck is 1.5 times the body length. It flies strongly with a down stroke that elevates the body with legs extending far beyond body. Due to its relative length, the neck is deeply bowed when it is flying with its head pulled back.
It is distinguished from the white form of the Great Blue Heron by its smaller size, smaller, slighter and very slightly down curved (not straight) bill, dark (not pale) legs, lack of head plumes, and its substantial back plumes in breeding plumage. It is distinguished from the Snowy and Little egrets by its larger size, yellow (not black) bill except in courtship, dark (not yellow) feet, relatively narrower wings, more upright alert pasture (McCanch and McCanch 1991). It is distinguished from the Intermediate Egret by its somewhat larger size, relatively longer (not stubby) bill, relatively longer, thinner more kinked neck, relatively longer legs, lack of head plumes, and the line of its gape (extending beyond not ending at the eye). It is distinguished from Cattle Egret by larger size, height, long neck, and lack of buff color. It is distinguished from the Eastern Great Egret by the latter’s combination of two tone brown to grey black legs and yellow bill and in breeding by a combination of black bill, bright green lores, pink to purple red wash on dark legs.
Systematics
The taxonomic position of this species has been long debated. For some time this indecision was resolved by placing it in its own genus, Casmerodius, laying between Ardea and Egretta. Morphological, behavioral, and biochemical evidence all point to these birds being Ardea (Payne and Risley 1976, Sheldon 1987, Sheldon and Slikas 1997).
Geographic variation among the great egrets has not been thoroughly investigated using molecular techniques. Based on DNA-DNA hybridization, the eastern population is recognized as a species, having been found to be as different from the North American form as it is from the Intermediate Egret (Sheldon 1987, Sheldon and Whittingham 1997). Similar analyses are needed to compare all populations. Attention especially needs to be focused on the area of potential overlap in north China and Japan between Ardea alba alba and Ardea modesta (Amadon and Woolfenden 1952).
Range and status
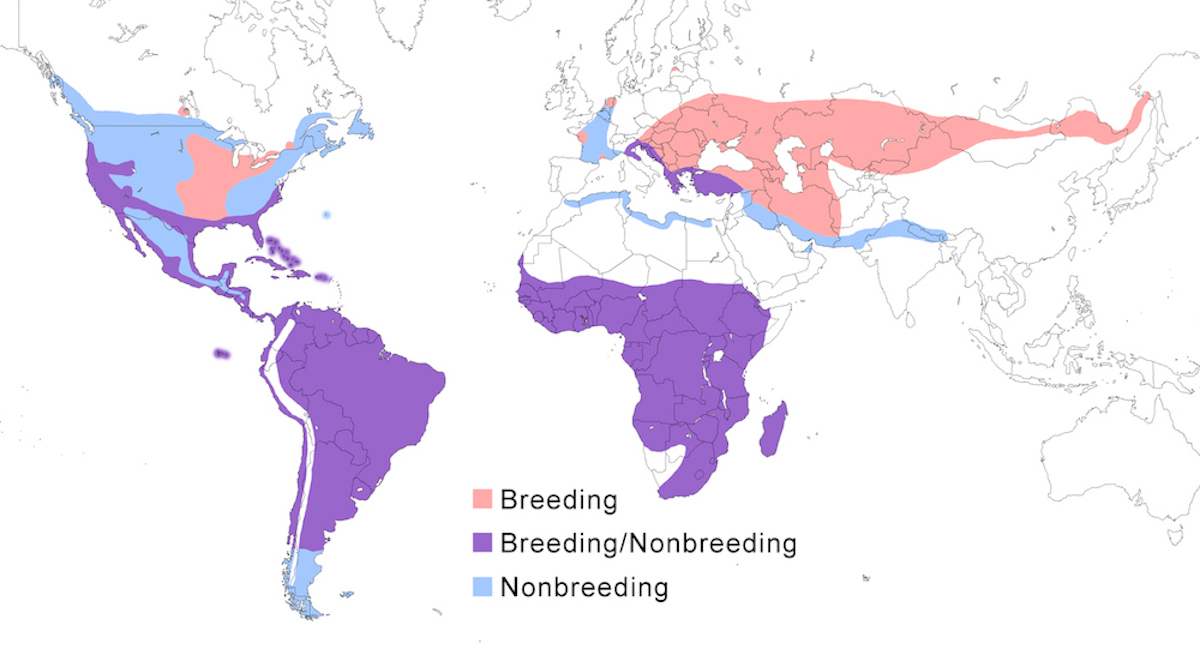
The Great Egret occurs through most of North, Central and South America, east Europe, Africa, and north Asia, avoiding deserts.
Breeding range: In the Americas, egretta nests to the north United States and south Canada in central Washington, California, Nevada, and Arizona, Texas, south Idaho, south east Saskatchewan, south west Manitoba, South Dakota, central Minnesota, Wisconsin, south Michigan, Ohio, south Ontario, south west Quebec, to Maine, central and eastern United States (Pratt 1983, Scharf 1989, Sauer 1991). They sometimes occur north of their breeding range in summer, as for example in Alaska (Gibson et al. 1987). It nests in coastal Mexico, Bahamas, Greater Antilles, north Lesser Antilles, Central America, South America to south Chile and south Argentina (44° S) and the Galapagos Islands (Nores 1986).
In Eurasia, alba nests in Netherlands, Latvia, France, Italy, Austria, Bulgaria, Romania, Poland, Ukraine, Hungary, Slovakia, Croatia, Albania, Greece, Moldova, Turkey, Iran, Turkmenistan, Uzbekistan, Afghanistan, Kazakhstan, south Russia (to Ussuriland), Mongolia, north China (north west Heilungkiang, north Simkiang (Voslamber 1992, Piacentini 1993, Marion and Marion 1994, Gavris’ 1994, Volponi and Emilianai 1995, Shernazarov 1995, Schogolev 1996, Van der Kooij and Voslamber 1997, Pugacewicz and Kowalski 1997).
In Africa, melanorhynchos nests from the southern tip of Mauritania, Senegal, Mali, Sudan, south Somalia, Ethiopia, Kenya, Tanzania, Zimbabwe, to South Africa (Baccetti 1983, Wallace et al. 1992, Turner 2000, O. Messaoud pers. comm.). Actual breeding records are scarce over much this African range. It also occurs on Madagascar, the Comoro Islands and Pemba Island (Cheke and Diamond 1986)
Nonbreeding range: The Great Egret spends nonbreeding season fairly far north in North America and south in South America. It occurs north to coastal Oregon, north California, central Nevada, central Utah, central New Mexico, central Texas, sometimes further (McMahon 1984). Most birds winter on the Gulf of Mexico coast and Florida, but occur on the Atlantic coast as far north as coastal Maryland and as Far East as Bermuda. Birds occur in nonbreeding season in the Bahamas, through Mexico and rest of Central and South American, south of the breeding range to the Straits of Magellan and Falkland Islands.
European birds spend the nonbreeding period around the Mediterranean Sea, Red Sea, Persian Gulf, in France Italy, Slovenia, Croatia, Albania, Greece, Turkey, Egypt, Tunisia, Morocco (Biondi et al. 1994, Vogrin 1999), but increasingly also further north (Bousquet 1998). West Asia birds winter in Iran, Iraq, and Pakistan, straggling to India. There is also a record of a Russian bird in sub-Saharan central Africa but there is no evidence of a regular influx of northern birds to the area and no evidence of a transSaharan migration by alba (Turner 2000). East Asia birds winter in south Tibet, north India, and south China.
In Africa, melanorhynchos numbers fluctuate seasonally suggesting population movements (Brown et al. 1982. Great Egrets winter in west Africa (Gatter 1988) but the origin of these birds is not clear, and likely not Europe. Birds spend nonbreeding season in South Africa and also occur in the Seychelles.
Migration: Northern populations of egretta disperse and migrate in complex ways. The two processes are not totally distinguishable. After breeding, egrets disperse in all directions and are regularly particularly reported north of the usual breeding range. In South America they occur south of the breeding range. They also disperse to nearby and oceanic islands. Dispersal records include south Alaska, Northwest Territories, north west and central British Columbia, south Alberta, south Saskatchewan, south east Manitoba, south Quebec, Newfoundland, Hawaii, Tierra del Fuego, Falkland Islands, Tristan da Cuna, Hawaii and Kuri Atoll (Byrd 1978, Pyle 1984, Gibson et al. 1987, Sirois et al. 1991, Tobish and Isleib 1992). The Atlantic coast population moves along the coast to the Bahamas and Antilles. Egrets from interior and Midwest North America move to or through Florida. Western North American egrets migrate to Mexico and Central America, although some remain farther north.
Alba disperses in all directions after nesting July to September, followed by a southward migration from late September to early November (Voisin 1991). On dispersal, it occurs in England, Wales, Scotland, Ireland, Belgium, Netherlands, Scandinavia, France, Spain, Malta, Germany, Switzerland, Poland, Czech Republic, Algeria, Morocco, Libya, Mauritania, Sao Tome, Cape Verde Islands, and Canary Islands (Stawarczyk 1984, Cotton and Murphy 1985, Manson 1985, Donovan 1988, Eccles 1988, Holmstrom 1989a, Dolton 1991, DeJuana and Ferrer 1996, Hazevoet 1999). The population is partially migratory with a few birds remaining in some breeding areas, at least in mild winters, a phenomenon that has been increasing in recent years (Festetics and Leisler 1999). . Birds from Austria and Hungary winter in Italy, the Balkans, Albania and Greece. Some birds move further south to Turkey, Israel, Egypt, and north Africa.
In west Asia, birds move to Iran and Iraq. In east Asia, birds move from north China. through east and west China to winter in south Tibet and south China. Birds from Korea and north Japan move to the Philippines. Return migration is in late February to early April.
In Africa, melanorhynchos has a substantial post breeding dispersal or short distance migration (Brown et al. 1982). Some birds appear to move south in migration to South Africa. Great Egrets winter in numbers in west Africa (Gatter 1988), but the origin of these birds is not clear.
Status: In North America, the species has been expanding its range northward in the past century, most recently beginning breeding in southern Ontario (Butler et al. 2000). Nesting and wintering populations are high in North America and increasing in Louisiana, Texas and California, although perhaps decreasing locally in the southeast United States (Fluery and Sherry 1995, Butler et al. 2000). In mid 1970’s over 110,000 nesting birds were censused in east and south United States. In the 1980’s over 43,000 nested in Louisiana (Martin and Lester 1991). It also is abundant further south with over 27,000 pairs reported in Mexico (Tabasco) (Scott and Carbonell 1986). 2,700 birds were counted in Honduras and Nicaragua (Frederick et al. 1997) In Costa Rica, 2,650 pairs were counted in a colony and 1,000 pairs along the coast of Surinam. It is common and probably increasing in South America (Morales 2000).
Populations of alba in western Europe decreased in 19th and early 20th centuries due to plume hunting. Recovery is occurring. It has expanded its nesting range and nesting and wintering population sizes in west Europe; nesting becoming established in Netherlands (1977), Latvia (1977), Italy (1992), France (1994) and Poland (1996) (Voslamber 1992, Celmins 1992, Marion and Marion 1994, Kayser et al. 1994, Volponi and Emiliani 1995 DeJuana and Ferrer 1996, Marion 1997, Van der Kooij and Voslamber 1997, Pugacewicz and Kowalski 1997). Its regional populations are increasing and total numbers in Europe are now 12,900-17,500 breeding pairs (Carpegna et al. 2000, Marion et al. 2000).
It is common, although patchy, in Africa. At its north limit in west Africa, about 150 pairs have been recorded in the Mauritanian portion of the Senegal delta (H. Hafner pers. comm.). About 3,000 pairs were counted in Niger inner delta Mali, 1,450 pairs in Senegal, 1,000 birds in a colony in Kenya, up to 3,800 birds in Zambia, 200 pairs in the Okavango in Botswana, up to 500 birds in Transvaal South Africa (Skinner et al., 1987, Turner 2000). It has been common in Madagascar where large flocks of 1,000 or more birds have been seen but breeding colonies appear to be declining. The resident population on the Comoros Islands is 50-100 birds (Turner 2000).
Habitat
The Great White Egret is a bird at home in a variety of wet habitats. It occurs typically in marshes, damp meadows, swamps, river margins, lake shorelines, flooded grasslands, and saltpans. It is also found in marine habitats such as tidal marshes, sea-grass flats, mangrove swamps, coastal lagoons, and offshore coral reefs. It uses human altered environments and agricultural lands including drainage ditches, rice fields, crawfish ponds, aquaculture ponds, pasture, and dry seashores. In some areas, fishponds, rice fields, crawfish ponds, and winter-rape fields have become critical habitats in the year-round status of the species (Ern 1990, Fluery and Sherry 1995, Grull 1998). It breeds in a variety of situations in trees, bushes, bamboo, reeds and other plants near water and on islands, sites that are protected from ground predators (Festetics and Leisler 1999). It occurs mainly in low lands, but can occur up to 1,800 m in Russia and 3-4,000 m in the Andes.
Foraging
The foraging ecology and diet of the Great Egret has been well studied through most of its range (Kilham 1980, 1984, Reichholf 1983, Rodgers 1983, Wiggins 1991, Ramo and Busto 1993, Austin 1995, Havens et al. 1996, Miranda and Collazo 1997, Dimalexis et al. 1997, Smith 1997, Grull 1998, Kasoma 2000). The Great Egret feeds in groups but more often feeds alone. It feeds almost entirely during the day, most actively near dawn and dusk; and in tidal environments it feeds according to tide stage, principally on outgoing tides (Austin 1995). Egrets roost in trees when not feeding and in communal roosts at night.
It principally feeds by Walking Slowly, which may occupy 60-90% of its time (Rodgers 1983). It usually feeds in shallow to moderately deep water, on shore next to the water, or on dry ground. It generally Walks in Erect posture with its head and neck well extended at about a 45o angle. It stops Walking when it sees a prey item. It also Stands next to places where prey is active. Its Crouched posture, usually assumed when a potential prey item is seen, involves holding its body horizontal and its head retracted. When examining a spot for prey, it may use Head Tilting, Peering Over, Head Swaying or Neck Swaying. It moves between feeding spots by Walking Quickly or Hopping. It uses Foot Stirring, Foot Paddling, Wing Flicking, Gleaning insects off plants and aerial techniques including Inplace Hopping, Hovering, Dipping, Plunging and Foot Dragging (Shanley 1984). Sometimes behavioral sequences can be quite complex. Catley (1983) reported that an egret Standing in the water began Foot Paddling while flapping its wings violently and periodically Hopping from the water; stabbed at prey apparently brought to the surface by this activity.
When feeding solitarily it will vigorously defend its site, using Upright and Forward displays, Supplanting Flights, and actual attack (Ryan 1999). It also is highly aggressive in flocks, defending its feeding area, supplanting nearby birds (usually after seeing the bird capture something), robbing other birds or being robbed. Robbing is common when birds attempt to handle relatively large prey (Kushlan 1978b, Kilham 1984, Smith 1989, Kasoma 1995). It also feeds by following other animals or using them to find feeding sites (Kilham 1980, Peter 1993, Tyler 1993, Berezovikov and Gistov 1994, McCall 1995, Sueur 1998).
In foraging, the Great Egret is not very efficient, having a fairly low rate of success per attempt, and not a particularly high strike rate (Rodgers 1983). Immature birds are even less efficient, in Florida averaging a 9% success rate.
Overall diet is diverse, but its principal prey in most situations is fish. In other situations insects or shrimp predominate. Insects include water beetles, water bugs, dragonflies, crickets and locusts. Its diet includes frogs, lizards, snakes, small mammals, and small birds. However, small prey dominates the diet (Mock 1985, Berezovikov and Gistov 1994).
Breeding
The breeding biology of the Great Egret has been well studied (Palmer 1962, Pratt 1970, 1972, McCrimmon 1974, Tomlinson 1976, Wiese 1976, Maxwell and Kale 1977, Mock 1978, Voisin 1983, Pratt and Winkler 1985, Wallace et al. 1992). Temperate birds breed in the local early spring and summer, April–July in most situations. In more tropical situations rains are more important than solar season and breeding varies from place to place and even from year to year. The Great Egret usually nests in the part of the rain cycle in which food becomes maximally available. This can be during the rains (Kenya, Ethiopia), end of the rains (Nigeria) or drying season (south United States, Mexico, Nigeria).
This egret nests in reed beds or in trees or bushes, usually isolated stands near water or on islands. Along tropical shores mangrove-lined trees are often used, as are willows Salix in many inland areas. Reed beds are particularly important habitats in some areas, such as east Europe (Schogolev 1996). The Great White Egret may nest either solitarily or colonially with other species, sometimes in colonies of over 1000 nests. The race alba tends to nest alone, whereas other races tend to be more highly colonial. Nests may be close together, less than 1 m apart or even touching. They are more spread out in reed colonies.
The nest is 80-120 cm wide, 20 cm high, made of sticks and lined with finer material. Nests from previous years may be re-used. Depending on substrate, nests may be placed 1 to 15 m above ground, tending to be placed high relative to other, smaller heron species.
The male, after a flying around period, chooses a display and nesting site. The male occupies the site Standing (with hunched neck and aigrettes fanned over the back), Twig Shaking, giving the Advertising Call, and building the beginnings of a nest. The site is defended vigorously by the male and also later by female using Upright (with sleeked plumage), Arched Neck and Forward displays, Supplanting Flights and attacks. The Forward includes having the body horizontal with head and neck retracted into an “S” shape, wings partly spread, rapid Tail Flick, extreme erection of the back plumes and head and breast feathers; Eeiee and Kraak calls may be given.
The males advertise using the Stretch display, in which the egret bends its fully extended neck slightly (not markedly) backwards while pointing its bill skywards; back plumes (but not head and neck feathers) are erect and fanned; the neck is slowly retracted neck while bending legs. Upon completion of the retraction the male stands tall again. It often followed directly by the Snap display, which is directed upwards. The Stretch-Snap combination is highly attractive to other birds. The male also advertises with Twig Shaking (also called Bow) in which it lowers its head and grabs a branch or nest stick sways from side to side and crouches shallowly. Advertisement also includes much flying around the colony, Standing, the Frawnk call, Circle Flights. Preening, Back Biting and Wing Touch occur after pair-formation. The Greeting Ceremony includes erection of the display plumes and raising of the wings.
The eggs are pale blue. Size differs geographically, averaging 61 x 43 mm and 56 x 40 mm in two studies. The clutch size is usually 3-5, range 1-6, being smaller in the tropics. Some averages are 2.97 in California USA, 2.4 in Australia, 3.4 in Kenya, 3.7 in Zimbabwe, 3.1 in South Africa (Pratt and Winkler 1985, Marchant and Higgins 1990, Brown et al. 1982). Incubation, by both parents, lasts about 25-26 days. Incubation starts with first or second egg, perhaps irregularly until the third egg. Young are semialtricial and nidicolous and hatch asynchronously. They are brooded and attended by both parents for times that appear to vary, from 15 to 30 days have been recorded, possibly related to food availability (Voisin 1991). In hot climates, parents shade the young.
Feathers begin to emerge at 7 days. They are feathered except for thighs by 15-21 days. The young start clambering from the nest in 21 to 30 days and leave colony in 42-60 days. Young are fed by regurgitation in the nest, later the chicks grab the adult’s bill, and eventually young come to the arriving parent to grab food. Brood reduction is the rule in Great Egrets (Mock 1985, 1987, Godfray 1986, Mock et al. 1987). High levels of aggression occur between older and the youngest chicks, with the younger generally dying. Aggression is less in two chick broods. Aggression levels are not directly related to food availability but chick survival is related to the amount of food, and perhaps type of food, delivered. Chicks appear to have a critical growth point (which may be variable due to food supply at 2-4 weeks). At this point the ability of the parents to supply food becomes critical (Voisin 1983, Pratt and Winkler 1985). In Europe, family groups are reported to stay together after fledging.
Hatching success tends to be high, baring catastrophic events, hatching rate in one study being 77.6 % (Voisin 1983). Late hatched young typically die of starvation or are killed. Fledging success is annually variable, 0.03 to 2.04 young fledged/nest, and averaging 0.90 over one long-term study in California USA. Post hatching, deaths of older young prior to fledging are generally due to predation or starvation. Colony wide reduced nesting success is usually due to adverse rainfall and water level conditions on the foraging grounds (Frederick and Collopy 1989a, Smith and Collopy 1995).
Population dynamics
Juveniles probably start to breed coming up on their second birthday. First year morality is 76% and adult mortality is 26%. Longevity is 22 years. Mortality is high in the first year probably due to the difficulty of learning to feed and also initial migration and dispersal risks. Thereafter, annual risk decreases. As a long-lived bird, local population size does not depend strongly in the short term on annual reproductive success (Pratt and Winkler 1985).
Conservation
The Great Egret, as currently recognized, is extremely widespread and has locally large populations in places throughout its range. In North America, recent range expansion and high populations in the south suggest it is secure there. In South America, the species is well distributed and stable as for as is known. In west Europe the historic range is being reclaimed (and expanded). Great Egrets are common in Africa, where large flocks of 1,000 or more birds have been seen. There is a concern in Madagascar that breeding colonies are declining due to human plundering, but impact on the egret’s population is less than clear as large colonies are likely being abandoned for smaller ones (Turner 2000). Throughout the range, the most critical conservation issue is the identification, protection, and management of important nesting sites and associated feeding grounds, and the identification of important stopover and non-breeding feeding areas. Colony site conservation includes protection, control of disturbance, and vegetation management (Obradovic 1988, Rodgers and Smith 1995). Conservation of feeding areas includes management of hydrology, salt intrusion, contaminants, and disturbance (Pyrovetsi and Crivelli 1988, Maccarone and Parsons 1994, Puglisi et al. 1995a).
Artificially maintained sites are critical to many population segments. Appropriate conditions need to be provided in order that these habitats continue to support populations (Elphick 2000). The establishment of the Great Egret in the Netherlands was in response to the creation of polder, its declaration as a nature reserve, and subsequent active management in favour of the species (van der Kooij and Voslanber 1997). Northern wintering is attributed to an increase in agricultural land (winter-rape) (Grull 1998). Colony sites can be actively managed on artificial sites and reintroduction programmes can be successful (Pullin 1987, 1990). In aquacultural situations in North America and elsewhere, the Great Egret comes into conflict with humans. Resolution of these conflicts through changes in aquacultural practices is essential to the conservation of the species (Mott and Flynt 1995). Excluding Great Egrets from aquacultural and other man-made situations such as reservoirs and farm ponds may not be me best conservation strategy. Aquacultural sites have become important feeding sites in some areas, likely maintaining population stability (Fluery and Sherry 1995), probably by reducing winter adult mortality. Maintenance of current population levels may require continuation of these practices and access of egrets to them. Great Egrets accumulate and are affected by persistent contaminants (Ohlendorf and Marois 1990, Spalding et al. 2000a). Clutch size, for example, was smaller prior to the ban of DDT (Pratt and Winkler 1985).
Research needs
The geographic species and subspecies limits of the Great Egret are no longer clear. There is a need to study geographic variation with respect to morphological, behavioural, vocal, and genetic differences and similarities. Among the questions are: What are the relationships among populations of Great Egret? How different is the North American form from the European or African forms? What is the breeding distribution and taxonomic status of birds in east Russia, north China, Japan, and Korea? Little is known about dispersal and survivorship. Additional migration and survival studies should be undertaken in major colonies dispersed across the species range. Study is needed of the foraging ecology of the species relative to its overall energy capture strategy. It would be worthwhile to better understand how feeding behaviour, aggressive behaviour, and sociality interact within this strategy.
Overview
The Great Egret is a successful heron. It has a large range, successful dispersal behaviour, and broad habitat preference, including an ability to use man-made feeding sites, a broad diet, and locally large populations. Its relatively long legs and neck and slow wading feeding behaviour allow it to feed in deeper water than the medium herons, but when appropriate also in very shallow water, on banks, or even on dry land. However, this apparently diverse approach to feeding habitat, behaviour, and acceptable food disguises a much more narrow and, at times, energy-limited niche. Despite feeding in many types of places, it often uses artificial situations. Despite possessing a large behavioural repertoire, it uses Walk Slowly and Stand techniques nearly all the time. Despite its long prey list, it feeds principally on a small fish and small insects. Feeding on small prey using a restricted feeding repertoire means that it has to capture many items to meet its daily energy demands. To accomplish this end, it feeds for exceptionally long periods during the day. So fundamental is its dependence on small prey that its nesting behaviour is characterized by internestling aggression and the (nearly obligatory) killing of younger chicks (Mock 1985a). Because the abundance and availability of small fish is determined in large part by water conditions, the Great Egret depends on finding feeding spots having appropriate tides or seasonal drying or relatively high productivity (Powell 1987, Kelly et al. 1993, Master et al. 1993, Gruell 1998). If it can defend these sites and feed alone, it does so. When it cannot, it joins other birds in locations of relatively high food availability such as drying marshes and tidal situations.
Despite feeding aggregatively, it seems best adapted to feed alone. Through most of the year, the egret preferentially does so. Even when nesting colonially, it tends to fly to feeding areas alone, choose vacant sites, and land away from other feeding birde (Smith 1995b). Feeding in aggregations does not increase efficiency, decrease capture variability, or increase prey intake, and takes more of its time in aggressive interactions (Wiggins 1991, Master et al. 1993). And it tends to capture even smaller prey in aggregations than alone. It is likely that aggregative feeding takes place because of the high availability of prey and despite the presence of other birds. Its aggressiveness, supplanting, and prey robbing appear to compensate for the flock disrupting the effectiveness of its Wading Stalking feeding behaviour.
While sufficiently successful for most of the year, during nesting the egret experiences a delicate balance between energy needs and its ability to meet these needs. Around mid nesting when there is an energy crisis and the young can easily begin arrested biomass growth that is usually fatal. At this time both parents forage intensively and it is then that brood reduction occurs, thereby determining reproductive performance for the year.