Grey Heron
Ardea cinerea (Linnaeus)
Ardea Linnaeus, 1758. Syst. Nat. ed. 10, 1, 141. Type by subsequent designation Ardea cinerea Linnaeus (Gray, 1840, List Gen. Birds, p. 66).
Ardea cinerea Linnaeus, 1758. Syst. Nat. 10(1), 143: Sweden.
Subspecies: Ardea cinerea jouyi Clark, 1907, Proc. U.S. Natl. Mus. 32, 468, Korea; Ardea cinerea firasa Hartert, 1917, Bull. Brit. Ornith. Club. 38, 6, Antinosy country, Madagascar; Ardea cinerea monicae Jouanin and Roux, 1963, Oiseau, 33, 104, Ile Arel, Banc d’Arguin, Mauritania.
Other names: The Heron in English; Garza Real in Spanish; Héron cendré in French; Graureiher, Fischreiher in German; Blauwe Reiger in Dutch; Серая цаплия in Russian; Gråhäger in Swedish; Cangak abu in Indonesian; Ao-sagi in Japanese; Cang lu in Chinese.
Description
The Grey Heron is a large grey heron having white and black accents, a white crown with black plumes, black belly, and white thighs.
Adult: The adult has a white head (including crown, sides, throat) with a broad black eye stripe extending from above the eye to the back of the crown and continuing as a crest with several elongated, black plumes. The long and heavy bill is yellow with a dull brown suffusion along the lower bill and top of the upper bill. The irises are yellow and lores are yellow turning darker green around the eye. The chin, throat, and neck are light grey to white, with the neck being tinged buff at its base. The foreneck is grey-white with two distinct broken black streaks running parallel down the median. The upper back and hind neck are pale grey, the lower back and upper wing blue-grey. Pale grey lanceolate feathers occur along the back. The flight feathers are dark grey to black contrasting with the paler upper wings and uniform grey to white under the wing. At rest, a black “shoulder” patch with a few white feathers is formed at the forward bend of the wing. The flanks are grey, the sides of the belly black, but the rest of the under parts are light grey to white, including the feathered thighs. The breast feathers are loose and elongated. The tail is grey. The legs and feet are green-grey to yellow-brown, varying in shade with age and season; the upper leg is paler (more yellow) than the lower leg.
During breeding, the black crest plumes attain full development and long white lanceolate plumes develop along the back, lower foreneck and breast. The iris, bill, and legs flush deep orange to red. The bill may retain some of this color until after the young have hatched.
Variation: The sexes are identical in plumage, but the male averages larger (Boev 1987a, b, c). Considerable individual variation can exist, including birds that are various shades of grey, white, black, brown, or buff (e.g., Krotoski 1983, Sanders and Ebels 1998). Geographic patterns of variation are recognized taxonomically. The European-African race, A. c. cinerea, is as described above. The buff color on the neck decreases geographically from east to west, and the eastern race jouyi typically lacks the buff color and is markedly paler on the neck, upper wing feathers, and back plumes (Vaurie 1965). The island race firasa averages larger, especially in bill and legs than cinerea. The West African monicae is paler, looking sufficiently pale grey to white to be distinguishable from migrant A. c. cinerea (Erard et al. 1986). In the western Indian Ocean, Grey Herons breeding on various islands vary from firasa-type to cinerea–type (Penny 1974, Langrand 1990).
Juvenile: Juvenile Grey Herons are more uniformly grey than adults and lack black plumage highlights and ornamental plumes. They have a grey to dark grey crown. The chin is white and foreneck appears brown-grey. The upper parts are grey-brown and under parts grey with brown grey streaking on the foreneck. The soft parts are duller than in the adult's. Legs are dark grey, the upper legs being paler and tinged yellow-green. In the first autumn and winter the upper parts become more blue-grey. The feathers of the back and breast are moderately elongated. From the second autumn subadult plumage is assumed, differing from the full adult in having the forehead and crown grey instead of white and the black of the sides of the belly less developed. Juvenile plumages are retained into the second year and vary among individuals from relatively more juvenile to more adult.
Chick: The chick is brown-grey above, with notable head crest, and white below. Irises are yellow, bill grey, and legs green-grey.
Voice: The Grey Heron is a rather vocal heron. Its distinctive vocalization is the “Frarnk” call, a loud far carrying flight call. The “Go” call, rendered “go, go, go”, is an alarm call. The “Oooo” call is an aggressive call, also given in the Forward display. The Oooo call is also used in the Stretch display, becoming a more gurgling “oooo” when given by both sexes in the crouched part of the Stretch. At the breeding colony, the species is very vocal, uttering a variety of yelps, squawks and other, softer notes. The “Rwo” call is the male’s advertising call. The “Arre” call is a landing call, rendered “arre, arre”, tending to a clucking on alighting. The Snap Display ends in a “Clop”. A Greeting Display includes bill-snaps. Bill Clappering occurs among pairs during formation and when in contact.
Weights and measurements: Length: 90-98 cm. Weight: 1,020-2,073 g.
Field characters
The Grey Heron is identified by its large size, grey coloration, distinctive eye stripes, black “shoulder patch” (wing edge), black belly, and white thighs. At rest, the black shoulder patch is a useful field character; and in flight the leading edge of the wing and especially the carpal joint are noticeably white against the general blue-grey of the body and under wing. The flight action is slow, with wings bowed. When descending to a feeding area or to the nest, the Grey Heron can be very agile in parachuting downwards. These herons usually are seen alone or in small numbers either standing erect, or hunched over next to the water.
It is distinguished from the Purple Heron by its white head and lack of chestnut color. Juveniles and darker than normal adults are distinguished from Purple Herons with difficulty, and wild hybrids are reported (Passarella et al. 1999), but Grey Herons are larger and lack rufous brown coloration. The Grey Heron is distinguished from the Black-headed Heron by its white head, larger size, grey (not black and white) under wing color. Immature Grey Herons have lighter legs (skin and feathers) than immature Black-headed Herons. The Grey Heron is distinguished from the Goliath Heron by its smaller size and has a white or grey neck (not chestnut) in the juvenile. The Malagasy race of the Grey Heron is distinguished from the Malagasy Heron by being smaller and lighter and having a black shoulder patch. It is distinguished from the Imperial and Sumatran herons by being smaller and more brightly black and grey.
Distinguishing Grey from Great Blue and Cocoi herons where each is vagrant poses challenges (Gantlett 1998). Fundamentally, the Grey Heron is a smaller and slighter bird (about 60% of the Great Blue Heron and 40% of Cocoi by weight), with a smaller bill, shorter and light-colored neck, and lacking chestnut thighs and chestnut edging to the “shoulder patch” of the Great Blue. If a field comparison is possible, the Grey Heron is more similar in size and shape to the American Great White Egret, but heavier (the Great Egret being about 60% of the Grey Heron’s weight). The general appearance of the Great Blue is of a larger, longer-legged, longer-necked, thicker-billed bird with “warmer” color tones (derived from the venous to chestnut wash to the neck and chestnut thighs) contrasted with the flat grey of the Grey Heron. The Cocoi Heron is a starkly black and white bird, with the general appearance, size, and shape of the Great Blue, with white neck and thighs like the Grey Heron, but with an entirely black cap that may be paler on the forecrown.
Out of range identification of the three species is even more difficult among juveniles. All three have a brownish wash to their plumage, which is both variable and changeable, dark crowns, dark upper bill, and shorter plumes. Juvenile Grey Herons are quite pale, have grey thighs, strong black and white throat and breast streaking, light legs, and a white “shoulder patch”. They tend to have a lighter crown than the other species, although this is variable among subspecies, jouyi being lighter than cinerea. Juvenile Great Blues are rather darker (except for some in southern Florida), have a pale to intense rufous tinge to the thighs and “shoulder patch”, and grey and rufous throat and breast streaking. Thigh color, so important in identifying adult Great Blue Herons, is grey as in the other species. Juvenile Cocoi Herons are duskier than the others, with dusky streaked thighs and stripes on the throat; most importantly they have a distinct cap that is dark grey to black, paler on the forecrown, even in young juveniles. In all the species, adult plumage is assumed gradually over the first or second year with the grey of the crown diminishing very gradually, accounting for much of the variation to be seen, and much of the confusion in identification. The most important marks are the very dark and distinctively edged crown of the Cocoi Heron, the larger size and bill of the Great Blue Heron, and the thigh and “shoulder patch” coloration.
Systematics
The Grey Heron is a typical heron related most closely the other Ardea and the Egretta herons. Indeed, in many ways it typifies the heron family in nomenclature, dispersion, habitat, and behavior. This is the Eurasian form of the large, solitary heron type, which is replaced by similar species in other biogeographic regions – the Great Blue Heron in the Nearctic, Cocoi Heron in the Neotropics, and the White-necked Heron in Australasia.
Of the four subspecies recognized here, two distinguish extremes of clinal variation across Eurasia. The third subspecies represents an isolated island form, a taxonomic distinction with analogies in other herons. The pale Banc d’Arguin has a highly restricted distribution. Erard et al. (1986) proposed the interesting hypothesis that it is a remnant form, more closely related to populations of Asia and Madagascar, and worthy of specific rank. More typical Grey Herons nest in nearby Senegal, and an individual in Senegal was reported as showing intermediate features between cinerea and monicae (Baillon 1989, Dowsett and Dowsett-Lemaire 1993).
Other described subspecies are problematic. Grey Herons on islands in the Asia-Oceania region include the heavy billed birds described as A. c. altirostris, occurring in Sumatra and Java (Voous and van Marle 1988). Those on Comores have been attributed to A. c. cinerea, whereas those on Aldabra have been considered intermediate between cinerea and firasa (Penny 1974).
Range and status
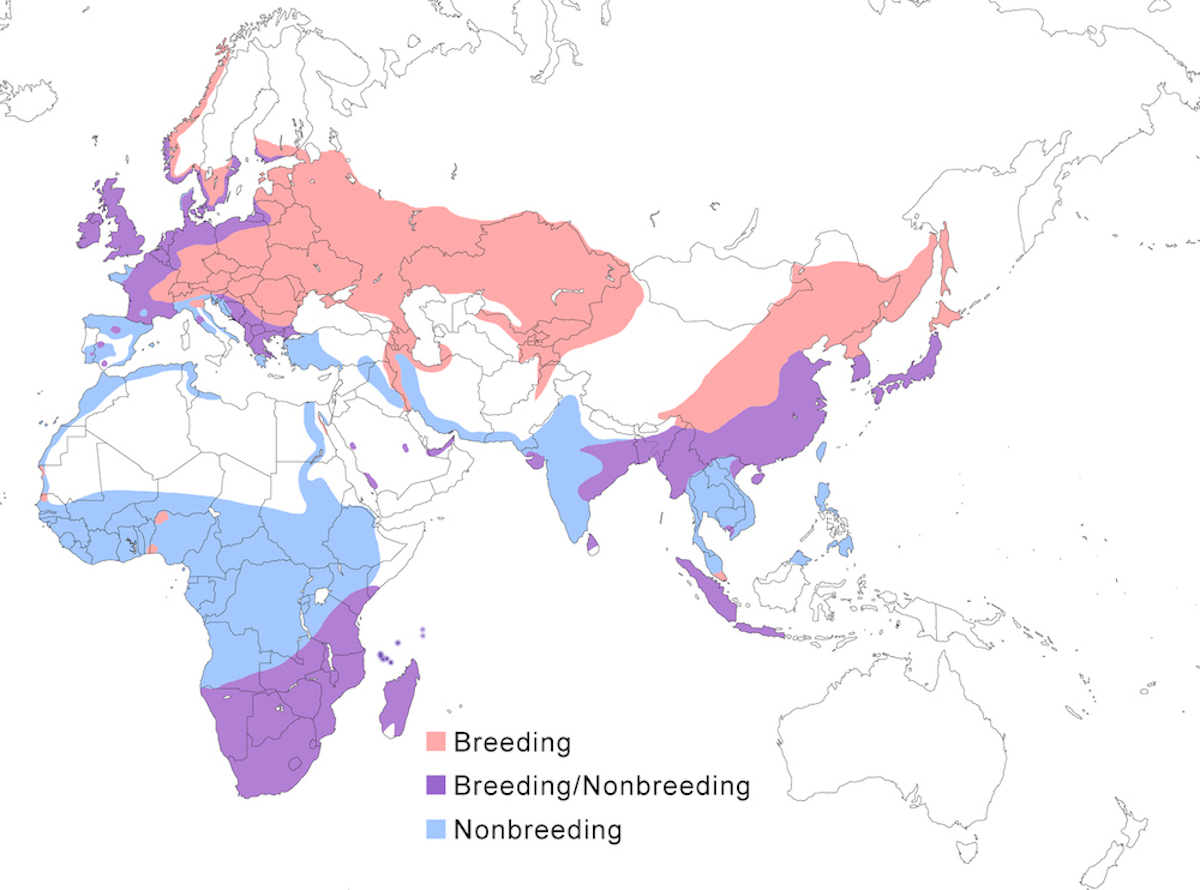
Breeding range: The breeding range of the Grey Heron covers most of the Old World south of the Arctic Circle, including Europe, Africa, Asia, and East Indies islands to Wallace’s Line.
A. cinerea cinerea occurs across northern Eurasia from Outer Hebrides, Great Britain (Bourne 1999), and Scandinavia, generally south of the Arctic Circle, through Russia (to Sakhalin). More to the south, it ranges from France, Spain, the Mediterranean, east Europe, Turkey, Iraq, possibly Israel (Ilani and Shalmon 1984), north Iran, north Afghanistan, Turkmenistan, Pakistan, India to Sri Lanka. Its status in Africa is intriguing. In north Africa, it nests rarely in isolated locations—Canary Islands, Cape Verde (Palacios and Barone 2001), Morocco, Algeria (van Dijk and Ledant 1983), and Tunisia. South of the Sahara and north of the Equator, small and sedentary breeding populations are known from Senegal, Mali, Niger, Côte d’Ivoire, Ghana, Nigeria, Sudan, and Ethiopia (not all mapped) (Turner 2000). Farther south, small breeding populations occur in Kenya, Tanzania, and Rwanda. They are resident throughout southern Africa, breeding patchily in suitable wet areas.
In the east Palearctic, the race cinerea integrates with jouyi in east Russia. This subspecies ranges from India, Mongolia (Stephan 1988), China to Hainan, Korea, Japan (Matsunaga et al. 2000), Myanmar, Vietnam and Cambodia (Mekong Delta-J. Tordoff pers. comm.), Malaysia (peninsular), Indonesia to Java and Sumatra (Danielson and Skov 1985, Silvius 1986).
The race firasa occurs in Madagascar. The race monicae breeds on islands of the Banc d’Arguin, Mauritania. It has been observed breeding elsewhere rarely, a few nests in the Aftout es Saheli located 100-200 km south of Banc d’Arguin (B. Lamarche through H. Hafner). Outside of Mauritania it has been reported only as far as Senegal.
Nonbreeding range: European nesting birds occur in winter in Great Britain, west and central Europe (e.g., Draulans et al. 1986a), even increasingly in northern Europe, such as in coastal lakes in Latvia (M. Strazds pers. comm.), the Mediterranean, Africa, and the Middle East including Saudi Arabia, and Iran. Asian nesting birds (Biondi et al. 1994) occur in winter in Pakistan (Balochistan, Sindh), India, south China including Hong Kong and Taiwan, south east Asia, Japan, Thailand, Malaysia, Philippines, and Indonesia (Borneo, Sumatra). Birds occur only in winter in Hong Kong, Taiwan, Philippines and Borneo (Sabah-Sheldon et al. 2001). Sub-Saharan Africa north of the equator is the important African wintering area for many populations (Turner 2000). This winter range generally is from Cape Verde Islands, Senegal and west Africa, south to 4° in Zaire, east to Somalia. Western European birds winter from Senegal, Guinea, Sierra Leone, Mali, Upper Volta, Togo, Nigeria, and Zaire. Eastern European birds winter in Mali, Upper Volta, Togo, Nigeria, and Ethiopia. Birds from Russia winter from Senegal, to south Egypt, to Kenya. There is no direct evidence of northern birds wintering south of the Equator but seasonal increases in birds in parts of Tanzania are suggestive (N. Baker and E. Baker in prep.).
Migration: Grey Herons disperse widely after the breeding season, this movement beginning generally soon after the young can fly. Dispersal may be in almost any direction, although mainly to the southwest in Europe. In southern Europe, postbreeding migration is in September and October, and prebreeding migration in February and March (Lekuona and Campos 1996c). In northern Europe, autumn migration is from early September to late November and return is in February and April (Grishchenko and Serebryakov 1993).
Birds breeding in Scotland and England are generally non-migratory (Partridge 1986), although British birds have also been recovered in Spain and Gambia (Christmas 1994). In southern Europe, some birds remain in winter but others migrate (Lekuona and Campos 1996a, c, Marion 2000). Migration is generally to the southwest in west and east Europe, to the southwest in west Asia and to the south and southwest in east Asia (Hancock and Kushlan 1984, Li et al. 1989, Round 1995). In the Mediterranean, migration is broad but corridors occur along both the eastern and western shores. Birds nesting in Africa, India and south east Asia appear to be sedentary.
Individuals sometimes wander widely. The Grey Heron is regularly seen in Iceland, Faeroes, and Ascension islands. In the New World, it has been recorded in Greenland, United States (Burton and Smith 2001), Barbados (Smith and Smith 1990), Tobago (D. Finch 2002), French Guiana, Azores, Trinidad (F. Hayes pers. comm.), Montserrat, Martinique, off Bermuda, Brazil (Para), and Venezuela (Marion 1988). The last six records are of birds that originated in France. It has been reported offshore in South Africa (Kirsch 1998). There are also occasional records from New Guinea, Kalimatan (Van Balen 1999), Australia, and New Zealand (Parkes 1974). Early sight records from New Zealand have been discounted (Dawson 1974).
Status: The Grey Heron is the most widespread heron in Eurasia, with a breeding population in Europe between 150,000 and 180,000 pairs (Marion 2000). In Britain, where the longest running census occurs (Bourne 1999), the population was nearly 6,000 pairs in 1991 (Carter 1992). The core of the European population is in France, Russia, Ukraine, Germany, the United Kingdom and Netherlands. With few exceptions, Grey Heron populations have increased markedly in numbers and range in Europe in this century. Northern expansion may be enhanced by post Pleistocene climate amelioration, but population increases are due principally to decreased hunting and depredation control (Moller and Olesen 1983, Marion and Marion 1987a, Bara 1988, Bakka 1989). In Britain, for example, the nesting population increased from 4,000 to 6,000 pairs between 1928 and 1991, rising at an annual rate of 8% per year during the 1980’s (Tomlinson 1992). In France, the nesting population increased from 4,500 to 27,000 pairs between 1974 and 1994, becoming the largest European population, due to protection (Marion 1997).
In West Africa, the nesting population outside Mauritania is small, perhaps less than 500 pairs (Turner 2000). The population in Niger averaged 8,500 (1994-7) (Brouwer and Mullie 2001). In east Africa, it is widespread but scattered. The resident population in Tanzania may be about 8,000 birds (Baker and Baker 2004). In southern Africa, the Grey Heron is increasing in Zimbabwe and South Africa, due principally to irrigation and reservoirs. Aquaculture has provided substantial additional feeding opportunities to which populations have responded (Kurz and Schmidt 1994).
The coastal Mauritania population is highly isolated, although the known population there has increased from 1,100-1,600 pairs in the 1960’s to over 4,188 in 1997 (Hafner et al. 1998b). The Malagasy subspecies is largely restricted to the western portion of Madagascar and is vulnerable owing to restricted range, exceedingly high levels of habitat alteration, hunting, and predation at the nesting colonies (Turner 2000). The Grey Heron is increasing on the Seychelles (J. Gerlach pers. comm.).
The Grey Heron is generally an abundant summer resident in China, Mongolia, Japan, Korea, and parts of Indonesia (Lansdown et al. 2000, Matsunaga et al. 2000). Breeding populations are small in Indochina and peninsular Malaysia.
Habitat
Grey Herons are generalists in habitat use. They are typically found in and around shallow water, generally along watercourses and shorelines, and usually in locations having roost trees nearby. They may occur in inland fresh waters, along estuaries, or in marine habitats. Shallow water, relatively large prey, and 4 or 5 months of an ice-free breeding season seem to be essential characteristics of suitable habitat. Among typical habitats are lakeshores, rivers and floodplains, reeds (Jeanmonod and Roulin 1989), swamps, ponds, mudflats, beaches, mangroves, and salt marshes. Dry or damp grasslands away from water are also used. Generally most abundant in the lowlands, Grey Herons can be found at surprising altitude, breeding to 2,000 m in Armenia and occurring to 4,000 m in northwest India.
Use of manmade habitats is the rule over many parts of its range. Rice fields and fishponds provide important feeding sites nearly range wide. Individual birds and even local populations may become dependent on these habitats and so are affected by changes in agricultural management, as well as other anthropogenic factors (Fasola 1986, Biondi et al. 1993, Thomas and Hafner 2000).
Grey Herons nest and roost in trees, with exceptions such as the Mauritanian population, so trees or other high vantage points are generally desirable. Some degree of isolation and protection is typical of places chosen for roosting and nesting.
Foraging
The Grey Heron usually hunts solitarily, but in situations where food is more concentrated, birds may feed in loose aggregations or even mixed species flocks. If conditions are especially favourable, quite large feeding aggregations may form, particularly after the breeding season. Herons feeding alone defend their feeding territories. Defence may be vigorous, and killing of intruders is known (Richner 1985). Aggression varies seasonally being most intense when young are being fed (Lekuona 1999). Sites may be near to the colony site or as far as 38 km away. When available feeding areas are poor and/or more distant from the colony, adults use two or three feeding areas and are no longer territorial (15% of breeders, Marion 1984, 1989).
Depending on prey availability and distance, herons use three types of feeding sites (L. Marion pers. comm.): (1) the individual defended feeding area composed of a single patch of about 20 ha, usually in wetlands or rice fields; (2) individual non-territorial feeding areas composed of two or three patches in poor habitats or in typical habitats more distant from the colony; and (3) neutral feeding sites used by many herons, where individual site appropriation is not possible. The latter are either ephemeral sites where high densities of food occur for short periods or sites too close to the colony and therefore attracting too many birds to be defended successfully. Herons appear to make trade-offs between nearer and distant sites, between using energy in territorial defense, or between site fidelity and exploring new feeding opportunities. Non-breeders and birds with little experience in an area spend more time exploring than do birds with local experience (van Vessem and Draulans 1987a).
Although considered in the literature to be typically a diurnal feeder, in fact Grey Herons feed at any time of the day. Generally, they feed most actively at dawn and at dusk and roost—usually in trees—during the middle of the day and at night. But they also feed at night, particularly during breeding when adults have to feed up to 23 hours a day to care for the young (Marion 1988). At fish farms, herons generally feed at early twilight or night, sometimes using artificial light but often feeding in the dark, where probably not coincidently they are out of sight of fish farmers who harass them during daylight (Draulans and van Vessem 1985a, Duquet 1987, Marion and Marion 1987a, b, Amies 1990, Carss 1993).
Time budgets actually observed depend on the interaction of food availability and food demand (van Vessem and Draulans 1987a, b). As noted, during the peak of nesting energy demand, birds feed nearly all day. In tidal situations, birds forage according to how tides make food available, selecting high or low tides (Matsunaga 2000). In France, they fed predominantly during the four hours of low tide (Lekuona 1999).
By far the most common feeding technique (used half or more of the time) is Standing, often thigh-deep in water, but also in shallower water, on land next to water, or on dry land away from the water. Walking slowly is used to move between Standing bouts or for slow exploration. Walking is done at a very slow pace. When fishing from a bank, herons frequently use Peering Over. They also may use more active behaviors such as Running with wings half-open, Swimming Feeding (for many minutes at a time), Diving and Plunging, but these behaviors are rather exceptional (e.g., Brown 1985, Guntert 1986, Laurenti 1986, Craney et al. 1989, Taylor and Carss 1990, Husband and Siering 1991). Despite its size, it feeds while in flight by performing aerial manoeuvres of surprising dexterity (Keighley and Hall 1995, Bowey 1997, Barbour and Barbour 2001).
Scavenging along the shore or in pastureland is not uncommon (Hiraldo et al. 1991), and it follows ploughs feeding on what is scared off or unearthed (Fox 1989a). In winter, it generally feeds in pastures on small mammals. The usual method of capturing prey is by a rapid Bill Thrust, preceded by retracting its head and neck. Pecking is used on dry ground, such as for earthworms. Food is nearly always dipped in the water, if available, before being swallowed. It is handled by biting, stabbing, and pounding to soften, to remove spines, to position for swallowing (fish are swallowed head first), or for breaking into smaller pieces.
Being a relatively large bird, it is not often harassed by other species on its feeding ground. In the air, it is usually able to avoid aerial attacks from other species by sideslipping, but is not always successful as there are instances of it being harried to its death by crows (Walters 1983). Being among the larger birds in mixed feeding situations, it can steal prey from other species such as gulls, cormorants, bitterns, and grebes (Cooper 1984, Sellin 1986, Kingston 1991, Hume 1992). In turn it is subject to piracy and other aggressions from smaller, more agile birds such as crows and gulls (Walters 1983, Warner 1986, Todhunter 1987, Skeen 1988). Intraspecific robbing is also common, especially among juveniles (Lekuona 2002b). It also feeds symbiotically with species such as mergansers and osprey (Laurenti 1990, Jacobs 1994).
The Grey Heron’s flexibility in diet and feeding behavior suggests it can make use of a complex set of foraging tactics in the face of different or varying prey abundance (Draulans and van Vessem 1985a, b, Draulans et al. 1986b, Draulans 1987, Draulans and Hannon 1988, Marion 1989, Peris et al. 1996). Evidence suggests that the species is highly opportunistic in prey selection. Prey do differ in profitability, depending on prey size, morphology, and behavior (Moser 1986b), and Grey Herons rapidly respond to changes in prey availability (Adams and Mitchell 1995). Fishing success of Grey Herons increases with increasing prey density, to a point, due to higher encounter rates and the use of more intensive hunting methods. Prey intake is highest when birds first arrive at a feeding site, declining with time. The ability of herons to adjust their foraging intensity, behavior, and expectations to ambiant conditions means that success rates turn out to be similar over different habitats.
During the non-breeding season, herons can easily change feeding to more productive sites in response to environmental conditions (wind, rain, clouds) that alter the sites’ relative value. However, during breeding season the territorial feeding strategy induces differences in feeding success between territories and thus adults, and directly influences the breeding success (Marion and Marion 1987a, Marion 1989).
Individuals differ in their behavioral choices, juveniles especially being less effective in both feeding and selection of the site and time for feeding. It is not clear how much of this ineffectiveness is due to juvenile choice or to dominance of adults at prime sites (Draulans 1987, Draulans and Hannon 1988). Juveniles, being less experienced, improve their foraging efficiency at fish farms more than do adults. Overall, juveniles appear to be less flexible than adults in the use of time, but more flexible in the use of space (Draulans and van Vessem 1985b).
That prey capture success, energy intake and frequency of feeding strikes of Grey Herons increase with higher prey densities indicates the importance of herons finding and feeding in locations that have high food abundance. At very high prey density, success plateaus out but the energy spent foraging decreases. Thus an important aspect of Grey Heron ecology is the ability to access sites supporting acceptable to high foraging efficiency. Diet variability follows, as herons change diet to take advantage of more catchable prey (Draulans and van Vessem 1987a, Adams and Mitchell 1995, Boldreghini et al. 1995, Peris et al. 1995). For example at a fish farm, herons focused on caged fish that were blind or in poor condition (Carss and Marquiss 1991, Carss 1993). Diet differs among colonies and among years (Draulans et al. 1987). Differential feeding success becomes exceptionally important during nesting. When feeding opportunities are distant and/or poor, for example if only frogs or voles are available, no more than two young can be produced, whereas if the feeding sites are rich, such as with eels, even if as far as 35 km away from the colony, herons can produce four young (L. Marion pers. comm.). Herons have more options for feeding site selection outside the nesting season, when energy demands are lessened.
Most studies have shown that Grey Herons do not seem to severely impact their prey supplies, taking only 6-8% of fish standing stock available in ponds (Draulans 1988a, Feunteun and Marion 1994), although effects may be greater in trout streams (Kramer 1984). These are crucial findings showing the well-being of local heron populations are often tied to their interactions with fish farms.
The Grey Heron takes a very wide variety of prey, the diet varying according to habitat and season (Draulans et al. 1987, Marquiss and Leitch 1990, Sawara et al. 1994). It is something of a specialist on larger prey, up to 19-25 cm long. Some may be much larger or longer. The size of prey eaten or attempted is often remarkable and at times this leads to swallowing problems (Davis 1982, Bentzien 1991, Geroudet 1993a, Cuenoud 1994). The typical strategy is to catch a few large prey during the course of the day.
Depending on location, fish or crustaceans tend to dominate a diet; amphibians, small mammals, reptiles, crustaceans, worms, insects, and small birds are also eaten (Fox 1989b, Bentzien 1991, Greaves 1991, Mienis 1991, Camici and Zimmerli 1993, Besson 1994, Cuenoud 1994, Carruette 1995, Hewson 1985, 1995, Lekuona and Campos 1996c, Kreuziger and Achenbach 1998, McCanch 2003). It is remarkably versatile and is reported taking such prey as muskrats, rats, grebes, wood hoopoe, frogs in winter, scavenged fish, and mussels. It also swallows a good deal of vegetable matter and even other foreign material (Hill 1988), but it is unclear if this is functional. It can scavenge carrion, dead fish, and scraps from fishing boats (van der Kelen 1993, Dies 1999a, b). Prey and size of prey varies seasonally, especially with respect to the food needs and food-handling capacity of young in the nest (Lekuona 1999).
Breeding
The time of nesting differs across the extensive range of this species. In temperate areas, the breeding season is restricted to spring and summer. In northern China, herons arrive in late March and in some years not until May. In coastal Russia (Maritime Province), herons arrive mid March and lay eggs in the first half of April (Litvinenko 1983). In Britain, nesting begins in February and March. In Spain nesting can be as early as January, corresponding to when minimum daily temperatures reach 8°C (Campos and Fernandez-Cruz 1991). Nesting in most situations is early relative to other herons, in Spain three months before other herons (Prosper and Hafner 1996).
In the tropics, the nesting season is more flexible, often occurring in the rains, the timing of which may differ from place to place. For example, in north India nesting is in July–October, but in south India in November–March. Birds breed at the height of the rainy season in Africa, from Senegal through west Africa, Sudan, Ethiopia, Kenya, Tanzania, and Rwanda (Turner 2000). The coastal monicae nest from April to December peeking in April–May (H. Hafner pers. comm.) Even in monsoon areas, nesting may start relatively early, well before the rains actually begin. In some tropical areas, nesting can occur nearly year round, such as in coastal Kenya, where birds feed in the littoral zone.
Most frequently these herons nest in patches of trees located where they offer some degree of isolation and privacy. They generally choose the taller of the tress available, up to 50 m, and in mixed colonies are the highest of the nesting herons (Fasola and Alieri 1992a). However, the total range of nest substrates and colony sites used is very large, including wood lots, rocky islands and city parks, herbaceous marsh plants, rock cliff ledges, buildings, walls and even the bare ground (e.g., Litvinenko 1983, Polasek 1991, Helm 1996). Nesting sites need to be within convenient flying distance of feeding areas. They fly 2-38 km to feed (Litvinenko 1983, Marion 1989).
The Grey Heron is primarily a colonial nester, although often in small colonies of 2-10 nest (e.g., Korvin 1987). The largest colony in Europe is 300-1,300 pairs (Feunteun and Marion 1994). Being the first nesters, their colonies attract other species such as ibis, spoonbills, cormorants, other herons (e.g., Commecy 1989, Carpegna et al. 1990) and especially corvids (Tkachenko and Shakula 1983). Heron nests are attractive to and are taken over by other species such as hawks (Boonman and de Vrieslaan 1992).
The nest is generally a stick platform with a grass liner about 50 cm in diameter, constructed from the most easily available material at the site. As noted above, nest sites vary widely, but they tend to be built toward the top of the canopy. While new nests may be so flimsy that the eggs are visible through them, pre-existing nests are often reused, being repaired and enlarged annually, becoming bulky masses. Windstorms can destroy nests, so they tend to be situated on secure tree branches. On bare ground, herons use twigs, herbaceous plants, and seagrass for nesting material (e.g., Litvinenko 1983). Males do more stick gathering than females and females more of the nest building (van Vessem and Draulans 1986c).
The courtship of the Grey Heron has been intensively studied (Witherby et al. 1939, Lowe 1954, Bauer and Glutz von Blotzheim 1966, Milstein et al. 1970 and others). The male on arrival at the courtship site or the colony site claims an advertising location, an old nest if available. In small populations, males may gather and courtship occurs at display sites prior to the birds moving as a group to the colony site (L. Marion pers. comm.). In larger colonies, there are generally several waves of bird arrivals over two or three months, and each group of new birds tends to nest together forming subcolonies (Marion 1988).
While displaying, through the day, the male repeatedly gives the Rwo call, its yelping advertising call. It also performs Upright displays with plumes erected, a bill-down version of the Stretch display, and the Twig-shake display. The male defends his territory against other males (and approaching females) mainly with Forward displays. The main pairing displays are the Stretch with up-pointed bill, combined with a milder version of the lunge movement or Bow-Snap display. Males Fly Around, and when alighting give a landing display, with powerful wing beats and arched neck with raised neck and head plumes.
Females approach the male, who is at first responds aggressively toward the intrusion giving Forward, Upright, and Stretch displays. As aggression wanes, Snap displays increase. After pairing occurs, Snap displays decrease, Stretch displays continue, as do much mutual preening, billing, and Bill Clappering. Pairs may Fly Around, and when one lands with a Landing Display, the other responds with Upright and Stretch displays. The individual permutations of the display repertoire can be complex, varying a great deal with age, temperament, and the stage of the pair bond. As the pair bond strengthens, the intensity and elaboration of displays diminish, particularly for males. Copulation occurs with the female standing and continues well into egg laying. At larger colonies extrapair copulations can occur, usually with neighbouring females (Ramo 1993, Lekuona and Campos 1998). The pair bond appears to last a single season only.
The eggs are pale green blue, relatively large but variable, 57-61 x 41-43 mm, laid at intervals of two days or longer. The clutch size differs with latitude. In temperate zones (Europe) clutch is usually 3-5 eggs (3-6 in Belgium – van Vessem 1991). In the tropics, egg clutches are reported to be 3-4 in India, 3-5 in Africa, and 3 in Madagascar. Complete clutches of 1 egg to 10 eggs are on record, but the latter seem unlikely to be attributable to a single female. Replacement clutches are reported, but this is less common than has been assumed because birds more typically mate with another bird or both leave the colony rather than renest (Marion 1988). Nests from failed attempts may be reused by other birds. In Spain early season losses led to a bimodal laying peaks, in March and again in April–May (e.g., Campos and Fernandez Cruz 1991).
Incubation takes 23-28 days, averaging about 26, but in tropical conditions may be only 21 days. Both parents incubate. They attend the nest for similar periods of time through the day (van Vessem and Draulans 1986b, c). Young hatch asynchronously, depending on the laying date. Young are fed upon hatching, so the earliest chicks become much larger than later chicks. This leads to differential survival, influenced by sibling competition, aggression, and even cannibalism (Litvinenko 1983, Lekuona and Campos 1993). In years of food shortage or other difficulties the smaller chicks do not survive, and in larger broods young chicks rarely survive irrespective of food supply.
The chick’s eyes open at hatching, and begging begins soon after. Parents brood young for up to 18 days. One remains on guard until about day 30. Thereafter, both parents forage at the same time. Both parents feed the young, which take food directly from the adult’s bill, except during the first days. When young cannot eat the sizes of prey most profitably caught by adults, the larger prey are removed from the nest by the adult, consumed, and predigested so that young are able to ingest them (Marion 1988).
Parents feed chicks first at the nest and then on nearby branches. Chicks that fall to the ground are not usually tended, although there are contrary reports (Lowe 1954). Feathers begin to grow about 7 days, and chicks are fully feathered at 28 days. Chicks remain in the nest for up to 10-20 days, where they spend most of the time standing, sleeping, begging, and tussling among sibs. They perform Upright, Forward and Snap displays, defending the nest. They can step away from the nest in 20-30 days, fly at about 50 days of age, and are independent at about 60-70 days. After fledging, generally juveniles are not fed or attended away from the nesting site.
Many factors affect the success of breeding. Egg failure can be high in some populations; in Belgium, egg failure was 17% (Campos and Fernandez-Cruz 1991, van Vessem 1991); nestling mortality was 33.3% (van Vessem 1991). In Spain, brood size averaged 2.2 nestlings from a clutch size averaging 3.5 (Fernandez-Cruz and Campos 1993). In Poland, brood size was 3.4 and 2.0 from a clutch size of 4.8 (Czapulak and Adamski 2002).
Success differs among pairs within a colony. For example, brood size increased with increasing age of parents, suggesting higher production by older, experienced birds. Breeding success was not affected by the number of birds present or by synchronization or by location in the colony. The most critical factor in nesting success is availability of food through the nesting season. Young also can be killed by predators, especially large aerial predators such as eagles (Berthelot and Navizet 1987, Naoroji 1990, Ytraberg 1992). Breeding success can be higher for birds using fish farms (Carss and Marquiss 1996). Nesting high in trees puts nests and nestlings at risk from windstorms (Lekuona and Campos 1995).
Population dynamics
The population dynamics of the Grey Heron are among the best understood of all the herons (North 1979, Marion 1981, Marquiss et al. 1983, Marion and Marion 1987a, Campos and Fernandez-Cruz 1989, van Vessem 1991b, Tomlinson 1992). Grey Herons generally do not breed until after the second year, although some frequent colonies as young yearlings.
Mortality is estimated to be 66.7% for yearlings (Fernandez Cruz and Campos 1993), which none the less allowed a population increase of 34.6% for 8 years prior to stabilization. The demography of the species is driven by its longevity (Cordonnier 1985), suggesting that adult survival is the most critical factor in population growth and stability. In Spain, the breeding population is skewed toward older adult birds suggesting saturation of the breeding population (Campos and Fernandez Cruz 1989, 1991, Campos and Fraile 1990).
Killing, hunting and accidental deaths of Grey Herons associated with humans can be a significant risk to stability (Kriedemann 1991). Century-scale population trends in Europe are attributable in large part to changes in its protective status (Marion 2000). Hunting is a particularly serious problem in some areas, such as Bavaria (Utschick 1983).
Fish farming has a conflicting influence. Populations can increase as birds, especially inexperienced young birds, feed at aquacultural facilities (Sawara et al. 1992, Samusenko 1993). However, mortality can also increase due to birds being killed at farms. In England water pollution has caused the deaths of breeding adults and eggshell thinning, but has had no obvious effects on total populations.
Winter survival is a critical factor for temperate populations. In Britain survival of both adult and first-year herons depends on severity of the winter (North 1978, Marquiss et al. 1983). The largest annual decreases in the British population occurred after the winters of 1947 and 1963. However, in Brittany, France, no difference in mortality rates was noted between hard and mild winters (Marion 1981). Young appear to be more susceptible than adults to winter kill (Tomlinson 1992).
The interactive effects of humans killing birds and hard winters have been demonstrated by Utschick (1983) showing that the potential exists for hunting to decrease populations to levels that inhibit recovery following a severe winter.
Range wide, as populations have increased in the twentieth under protection, hunting has decreased as a factor, range wide and density-dependent factors, such as territoriality, and also winter mortality have become more important factors in regulating population size (Marion 1984, Marion and Marion 1987a). It is likely that in much of Europe population size is becoming governed by habitat availability (Utschick 1983, Fasola and Alieri 1992b).
Conservation
The species is widespread, adaptable, and globally abundant. Overall, the species is secure. However, certain populations are of conservation concern. The Malagasy population (firasa) is threatened within its limited range by human activity and ongoing habitat destruction. It is considered globally Endangered by the Heron Specialist Group. Nesting sites require active protection and a local awareness campaign describing the critical nature of the bird’s situation should be undertaken. The population of distinctive pale birds from islands in the Banc d’Arguin, Mauritania (monicae) also has a highly restricted range. Given the population increase and protection offered by the national park, its current situation seems secure at present. Surveys and monitoring should be continued and the birds of this color form should be looked for elsewhere in west Africa. Populations on various Pacific Islands are small enough to warrant conservation concern. The Grey Herons of Sumatra (which have been called altirostris are considered to be globally vulnerable by the Herons Specialist Group. A known population numbering about 700 birds occupies restricted habitats under threat. A conservation plan for this population is needed. Populations of concern include birds of the Comores, Aldabra, and North Marianas. Surveys are needed to determine the true numbers of these populations, seasonal distribution, and migrations and location of important areas as well as their appropriate taxonomic status. Other populations of concern include that in Indochina (Thailand, Malaysia), where few colony sites are known, and that in the Danube Delta, which appears to be decreasing.
Through the rest of its range, where known, the species is either stable or increasing, in some areas spectacularly so. In west Europe it is likely at carrying capacity, its populations now being influenced by winter conditions and the amount of habitat. The European population had reached an extremely low level by the mid twentieth century due to killing by hunters and fishermen for the preceding century (Knief and Drenckhahn 1984, Marion 1984, 2000, Marion and Marion 1987a). In central Europe, for example, there was an increase after 1880-90 and then a decrease from 1930 to 1950. Legal restrictions that began in the 1950's, along with the increase in artificial feeding situations and general warming of the climate have been beneficial. The increasing heron population has brought it into renewed conflict (or the perception of conflict) with the expanded aquacultural industry. Through its known history, the species’ welfare has depended in large part on the outcome of its direct interactions with people (Cordonnier 1985, Marion 2000). These also are essential ingredients for management within a contemporary conservation strategy.
Among the factors under human control, the elimination of hunting and the killing of herons at fish farms are those that require immediate attention in conservation planning. For a long-lived bird demographically dependent on adult survival, it would be hard to make an argument that herons should be a huntable species for either cultural or food purposes. Renewed hunting poses a threat to the Bavarian heron populations (Utschick 1983). In Scotland 800 herons were estimated to have died in one year at fish farms (Carss 1994). Perhaps the only reason why the number of birds killed at aquaculture facilities has not reduced the overall populations so far is that it is probably young birds that are mostly killed. Such killing occurs despite excellent studies showing that the effects of herons on most fish farming operations are slight (relative to the full scope of mortality) or manageable through aquacultural practices (Utschick 1984a, b, Marion and Marion 1987b, Perennou 1987, Carss and Marquiss 1991, 1996, Carss 1993, Marion 2000). For example, at a fish farm with caged trout, herons selected small, blind or poor condition fish, and losses were small compared with overall losses, yet these appeared serious to farmers. Furthermore, in France, it was found that impacts to aquaculture were not caused by the local nesting birds, so reducing the local heron population would not have any effect on depredation (Cordonnier 1985). Utschick (1994a) found that to reduce heron presence at fish farms in Bavaria from 0.6 to 0.1 herons per day would require eliminating 75% of the population of Central Europe. Fishery management plans and heron conservation plans need to develop ways to minimize both the damage by herons at fish farms and the damage to herons at fish farms.
Timber operations are a constant problem through most of the range (e.g., Bracko 1985, Kneif 1986, Tallone 1991). Unless sensitively controlled, harvesting can remove nesting trees and the disturbance of nearby operations, even if a colony site is preserved, can be devastating to the nesting herons. Population increases in South Africa and Zimbabwe correlate with the establishment of reservoirs, dams, and irrigation areas (Turner 2000). This is an example of how the maintenance of current population levels requires maintenance of these artificial situations.
Research needs
The Grey Heron is one of the best known of the herons. As a result, the knowledge base is sufficient to indicate the direction of conservation actions. Studies of Grey Herons have also contributed significantly to understanding heron behaviour and biology in a larger sense. Important gaps exist in the biology, taxonomy, and conservation needs of peripheral populations, particularly island populations, in west Africa and the Indo-Pacific. These populations require detailed examination. Additional study in both the field and of specimens of monicae and island forms is needed to determine the range of variation in these populations and relationships among recognized subspecies and other populations. Given the adaptability and vagrancy of the species, observers should search for breeding occurrences near where vagrants occur. Its potential for establishment in North America and the Caribbean islands should be monitored. The research base showing the limited impact of Grey Herons on fish farming is strong. What may now be needed are studies of ways to further reduce what impacts do occur, namely a socioeconomic study of heron-fish farmer interaction, and sociological studies to find new approaches to achieve co-existence for herons and fish farmers. Demographic analysis is desirable to study conclusively the potential importance of adult mortality (especially from hunting and other killing) to population stability.
Overview
The Grey Heron is a highly successful species due to its biological flexibility. Its large size allows it to remain relatively far north in winter or to migrate as appropriate. Its large size also allows it to dominate other herons in defending feeding territories and to take a wide variety of prey types. It uses many habitats, from open seashore to secluded ponds and from fields to ditches and can take advantage of mancaused prey abundances, such as at fish farms. The Grey Heron tends to be a communal nester but a solitary feeder, a combination that takes advantage of both strategies. Herons can continue to feed in defended areas, but if superabundant food becomes available can shift to those sites and feed more aggregatively. The Grey Heron is one of the most abundant species over its large range, due primarily to its flexibility and adaptability.