Little Egret
Egretta garzetta (Linnaeus)
Ardea garzetta Linnaeus, 1766. Syst. Nat. ed. 12(1), p. 237: ‘in Oriente’, restricted to Malalbergo, near Ferrara, NE Italy.
Subspecies: Egretta garzetta immaculata (Gould), 1846: Northern portion of Australia (=Port Essington, Northern Territory); Egretta garzetta nigripes (Temminck), 1880: L’Archipel des Indes (=Sunda Islands); Egretta garzetta dimorpha Hartert, 1914: West Madagascar; Egretta garzetta schistacea (Hemprich & Ehrenberg), 1828: Red Sea; Egretta garzetta gularis (Bosc), 1792: Senegal River.
Other names: Lesser Egret, Spotless Egret, Western Reef-Heron, Western Reef Egret, West African Reef Egret, Indian Reef Egret, Arabian Reef Egret, Dimorphic Heron, Madagascar Reef-Heron, Mas- carene Reef Egret in English; Garceta Común, Garceta Sombria in Spanish; Aigrette garzette, Aigrette des Récifs in French; Seidenreiher, Riffreiher in German; Kleine Zilverreiger, Westelijke Rifreiger in Dutch; Sikeshäger, Revhäger in Swedish; Малая белая цапля in Russian; Kuntul perak kecel in Indonesian; Tagak (Tagalog) in Philippines; Ko-sagi in Japanese; Bai in Chinese.
Description
The Little Egret is a thin, medium, dimorphic (dark or white) heron, with a long thin neck and bill, dark legs and yellow feet (in most forms), and active foraging methods. When breeding, the bird acquires distinctive head, chest and back plumes and red lores.
Adult: The Little Egret is highly variable. Three coastal populations are dimorphic having both white and dark birds. Soft part colors differ geographically, with age, and with season. White forms have predominantly white plumage, although sometimes flecked with dark feathers. Dark forms are blue to grey with variable amounts of white, characteristically under the chin. Individuals with intermediate plumage are common. In nonbreeding, short plumes may occur on back and chest. Bill colors include yellow, brown or black. Legs are either yellow or dark, blue forms always having dark legs. The feet of most populations are yellow.
When breeding, the Little Egret develops characteristic plumes on the back of the head, lower throat, and back. Particularly distinctive are the two or three thin, lanceolate crown plumes, which can be up to 16 cm long. The elongated feathers at the base of the neck are lanceolate distally. Lax back plumes are long, exuberant, and slightly recurved, but do not extend beyond the tail. At the height of courtship, the bill is black and lores turn red, as do the feet in yellow-footed birds. The lores then fade through white to the yellow or blue grey color of nonbreeding birds. Other soft part colors may differ among populations or individuals (see below).
Variation: Males are larger than females and juveniles are smaller than adults (Nota 2000). The extent of individual variability, the interbreeding of birds representing different racial types, and dispersive tendencies that take birds outside their range confound patterns of geographic variation, and taxonomic characterization. Individual and populational variability involve morphology (bill shape, bill length, bill thickness, leg length) and soft part color (lores, legs, feet). Populations adapted to coastal environments, like other coastal herons, are dimorphic and tend to have thicker bills (Wassink 1979, Hancock and Kushlan 1984).
Egretta garzetta garzetta is all white. Its bill is straight, sharp, dagger like, and all black, usually shiny and sometimes with a bit of yellow at base of lower bill. During nonbreeding, the irises are yellow; the lores are blue grey; the feet are yellow with color extending up the back of the lower leg. During courtship, the bill remains black, however the lores become pink to red before fading to colorless. The feet also turn pink to red before fading back to dull yellow.
As far as has been proven, garzetta does not have a dark form, although this is highly debated. Dark birds have been reported periodically within the normal inland range of garzetta garzetta, dating back to 1869 (Ashkenazi 1993). A dark phase garzetta would have a thin black bill, grey to dark grey plumage, blue grey lores, and bright yellow feet. Whether such a bird would have a white throat is unclear. But some of these characteristics are within the range of variation of gularis and schistacea. The lack of specimens and molecular data and uncertainty in the underlying subspecific taxonomy have interfered with interpreting these dark birds. Racial hybridization is also a potential confusing factor. For example a bird recently appearing in Europe, interpreted by many to be gularis, is more likely the result of the pairing of garzetta and gularis (photo in Hancock 1999).
Nigripes is white. It is similar to garzetta except its feet are black not yellow although occasionally having yellow soles. Nonbreeding lores are blue grey becoming red in courtship. The irises become red, but the feet remain black.
Immaculata also is white. Nonbreeding lores are yellow, an important distinction from garzetta. Legs and feet are black, with yellow soles. In courtship, the irises turn red, as do the lores before reverting to yellow. The feet remain black although the soles can turn red. The back plumes are the most spectacularly developed of the races. Interestingly, the long-standing confusion about the identity of yellow-lored Little Egrets originated as far back as 1833 when Edward Lear in the Birds of Europe painted an Australian immaculata rather than a European garzetta (Hancock 1999).
In addition to the two all white subspecies, three of the named populations are dimorphic. These “Reef-Herons” are all coastal, a situation occurring in other herons that are dimorphic. Each has an all-white and a dark form, as well as intermediate-plumaged birds. The dark forms are blue, usually with a white throat. Some have some scattered white feathers.
Gularis populations are composed predominantly of dark birds, which range in color from grey black to pale charcoal grey with a distinctive white chin and throat. They often show a white patch on the leading edge of the wing and also a variable amount of white feathering on the upper wing. Light morph birds are rare, occurring mostly in the northern portion of the range and on Sao Tome Island (Turner pers. comm.). These birds are white but often have a varying amount of grey feathering scattered about. For both forms, nonbreeding lores are yellow to pale green. The relatively long and thick bill ranges from yellow to horn brown, but is not black. It is heavier and slightly more down curved than the bill of typical garzetta. This is a character difference that can be discerned in the wild as illustrated by Barlow and Wacher (1997). The legs are variably yellow brown to dark brown with dull yellow feet but never black as in garzetta. In courtship the bill darkens and the lores become red fading back to yellow. The legs become dark brown before returning to lighter shades. The feet become red, then return to yellow. Both morphs have two head plumes, but these are somewhat shorter than those of garzetta.
In dimorpha dark individuals are blackish, slightly lighter than gularis, usually with white throats and white wing patches on the carpel joint and sometimes with white speckles. Rare intermediate birds are paler, with mixed dark and light feathers. Light individuals are white occasionally with stray dark feathers. Nonbreeding lores are yellow. The bill and legs are jet black, and the feet are yellow. The bill in some populations is thicker than that of garzetta, although this is not universally the case such as in coastal east Africa (Baker and Baker in prep.). In courtship, lores and feet are red returning to yellow.
Schistacea is a very confusing situation. It has never been properly characterized except geographically. Birds allocated to this subspecies are highly variable. Indian birds were formerly distinguished as asha, suggesting the extent of variation. Light birds are white, often with dark mottling. Dark birds are blue grey with a white throat, occasionally with white wing patches. Nonbreeding lores are yellow, blue green, or even colorless. The bill too is highly variable, ranging from yellow to pale green to olive green to light brown to black. It may be somewhat down curved and relatively thick at the base (but again with considerable variation). The bill of some birds is darker toward the tip, sometimes to one third of its length. Legs vary in the same ways as the bill, but for any bird bill and leg colors do not necessarily correlate. The feet of most birds are demarcated from the darker legs, being yellow to dark green, but this is not universal. In breeding, lores turn red or orange. The bill can be yellow with a variably red to orange flush, turning to yellow brown after egg laying. Legs are black or brown, variably flushed orange. Feet become pink to red returning to dark green. The typical schistacea is a somewhat larger, heavier bird than garzetta.
Identifying and separating garzetta, gularis, and schistacea have been a matter of detailed discussion (Hancock 1986b, 1999, Grussu and Poddesu 1989, Ashkenazi 1993, Dubois and Yessou 1995). A typical garzetta tends to be slimmer, with longer, narrower, black (not variable) bill, blue grey lores, gregarious, and inland (not coastal). Cramp and Simmons (1977) suggested separating garzetta and gularis on the basis of tarsus to bill ratio, but due to variability this turns out not to be reliable (Ashkenazi 1993). Variability, including the intermediacy of many individuals, makes racial identification difficult. Such variation appears to be in part the result of intergradation among populations. The best example of this is in India, where schistacea birds have moved inland to nest in the same colonies as garzetta. The many examples of intermediate birds, presumably resulting from cross breeding, constitute strong arguments supporting the concept that these populations are not different species. Needless to say, geographic and polymorphic variation in Little Egrets is not yet sufficiently understood.
Juvenile: Juvenile plumage is extremely variable (Parasharya and Naik 1984). White juveniles may have varying amounts of mouse brown to grey feathers and can appear intermediate between pure color phases. Dark color birds may have white chins. Juvenile garzetta have green bill with black marking, pale green lores, and dull black and green legs. Juveniles lack or have poorly developed plumes.
Chick: Chicks of white forms have white down, with longer crown down forming a crest. The bill is yellow horn, legs and feet are green. Dimorphic races produce a range of white, grey blue, grey, and speckled chicks. There is much variation in bill color of the chicks (Voisin 1991). Bills generally become deeper yellow then brown then usually black. Feet and legs turn dark green then to brown with feet light green turning to yellow green. Chicks of dimorpha have relatively large legs and feet.
Voice: The Little Egret is highly vocal. The “Kark” and “Kre” calls, the latter rendered “kre, kre, kre”, and other variants are aggression and flight calls. “Aaah” call is taking off flight call and also used on feeding grounds. Landing call is “Da-wah”. Advertising calls are variable including, a distinctive gargling “La” call, rendered, “la, la, la, la, ah, h, h, h”, in which the syllables are interrupted. “Dow” call and “Po” call, each with 5-9 repeated syllables, are also Advertising Calls. “Ggrow” is an aggressive call. A gulping “Ow” call is used during the Stretch. Young call to parents with a “Ka” call, rendered “ka, ka, ka, ka, ka”.
Weights and measurements: Length: 55-65 cm. Weight: 300-710 g.
Field characters
The Little Egret is identified by its combination of thin body form, long neck (half of total height) medium size, (usually) yellow feet, and when breeding its crest plumes, chest plumes, and long back plumes, that do not extend beyond the tail. Flight is slow with intermittent gliding on rounded wings, head and long neck retracted, feet extending beyond the tail. It feeds actively.
It is distinguished from Cattle Egrets by longer, thinner, usually dark (not yellow) bill, black (not light) legs, slimmer body, longer neck, upright posture, and white (not buff) plumes. It is distinguished from the Squacco Heron by its larger size, upright posture, and white (not tawny) back. It is distinguished from the Intermediate Egret by being smaller, with yellow feet, dark (not yellow bill) and, during the breeding season, by plumes that stop at the tail (not extend beyond). It is distinguished from the Great Egret and Eastern Great Egret by being smaller, yellow feet (in most forms), and in nonbreeding plumage black to dark (not yellow) bill. The white form is distinguished from the white Eastern Reef-Heron by longer, thinner, dark bill (not light with black tip), and dark (not green legs). It is distinguished from the Snowy Egret in nonbreeding by larger size, longer bill, blue grey (not yellow) lores, flattened head shape), less vigorous feeding, and, in the breeding season, by the two long head plumes and the color of the feet extending up the lower leg (Murphy 1992, F. Hayes pers. comm.). It is distinguished from the Chinese Egret by blue grey (not blue) lores, black (not variably yellow to dark) bill, and, in breeding season, by fewer, simpler (not bushy) head plumes. The dark form is distinguished from the Black Heron by its lighter grey plumage, thinner bill, two crest plumes (lack of a shaggy crest), longer legs and the white throat. The dark form is distinguished from dark Eastern Reef-Herons by thinner bill, white throat (not just chin), longer legs, and upright posture.
Systematics
Hancock and Kushlan (1984) first proposed that several medium Eurasian egrets were best considered as representatives of a single polymorphic species. Since then, the concept that these are closely related species has seen common agreement (e.g., Sibley and Monroe 1990) whereas the more radical concept of conspecificity has been accepted by some (e.g., del Hoyo et al. 1992) and much discussed by others (Yesou 1984, McLaren 1989,Voisin 1991, Murphy 1992, Hancock 1984b, Ashkenazi 1993, Grussu and Poddesu 1989, Saleh and Mohamed 1990, Newman and Holtshausen 1990, Dubois and Yesou 1995).
The issue of the relationships within the little egret group, the classical Little Egrets (garzetta, nigripes, immaculata), the Dimorphic (or Mascarene) Reef Egret (dimorpha), and the Western Reef-Herons (gularis, schistacea including asha ), is far from settled and remains one of the more intriguing taxonomic and evolutionary problems in the Ardeidae. While the dialogue has been valuable, it has not produced definitive answers. We had hoped that in the interim between our books, additional quantitative studies would have explored this intriguing issue, and are disappointed to be unable to report new empirical evidence to either support or negate the taxonomic hypothesis proposed over 15 years ago. Morphological studies have not yet examined specimens of all races. Biochemical studies of all the forms have not been conducted. Behavioral observations are not more robust or more helpful than before. What is known is that variability is high, neighbouring populations are hard to distinguish unless differently colored, and these populations are known to interbreed, such as in Kenya (where birds appearing to be dimorpha, schistacea and garzetta all occur) (Turner pers. comm.) and in coastal India, the Philippines, and north Celebes.
In retaining the Little Egret as this polytypic species, we again urge definitive, range wide studies using a combination of biochemical, morphological and behavioral characteristics. Of particular interest are the areas where the forms appear to interbreed, such as inland Kenya, coastal India, Philippines, north Celebes, and Kisite Island (where birds appearing to be dimorpha, schistacea, garzetta occur).
Range and status
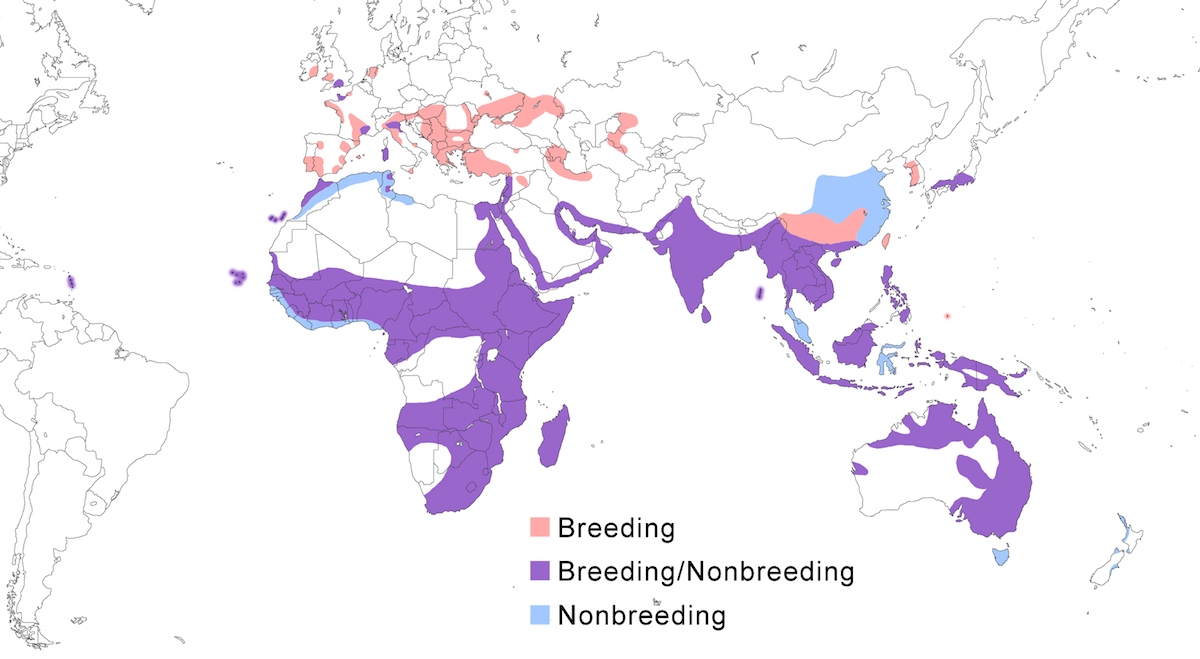
The Little Egret occurs over much of the Old World, and more recently into the New World. It occurs in Europe, Africa, Madagascar, Asia, East Indies, Australia, Pacific Ocean islands, and West Indies.
Breeding range: Garzetta is the widespread Old World form. In Europe, it nests in Spain including Canary Islands, Balearics Islands, Portugal, France, England, Wales, Ireland, Netherlands, Belgium, Italy (Sardinia), Germany, Czech Republic, Slovakia, Bulgaria, Austria, Hungary, Romania, Croatia, Yugoslavia, Albania, Moldova, Ukraine, Turkmenistan, Russia (to Volga Delta), Greece, and Turkey. In the Middle East, it nests in Turkey, Israel (spilling over into Jordan), Azerbiajan, Kazakhstan, perhaps Iraq, and Iran. In Asia, it nests in Turkmenistan, Uzbekistan, Pakistan, India, Sri Lanka, Nepal, Myanmar, east and south China (Guangdong, Guangxi, Yunnan, Szechwan, Hainan, Taiwan), North Korea, South Korea, Japan (north to central Honshu), Cambodia, Laos, Vietnam, Thailand, Singapore, Indonesia (New Guinea?), Philippines, Palau, New Britain, Australia (West Australia, Queensland, Victoria, Bass Straits Islands, Tasmania). In Africa, it nests in Egypt, Algeria, Tunisia, Morocco, Cape Verde Islands, Mauritania, Senegal, Gambia, Liberia, Mali, Ghana, Chad, Nigeria, and Gabon. Also in Sudan, Kenya, Uganda, Rwanda, Tanzania, Mozambique, Zimbabwe, Zambia, Botswana, South Africa, Namibia and Angola (Baccetti 1983, Fesenko 1984, c and Bohus 1990, Gustin and Pizzari 1992, El Din 1992, Eminov 1993, Le Dinh Thuy 1994, Smiddy and Duffy 1997, Leibl and Hagemann 1997, Schuster et al. 1998, Rajchard and Novak 1998, van den Berg 1999). It has recently become an established breeding species in Barbados in the Lesser Antilles, and is suspected of breeding in east United States, based on the observation of a likely hybrid Little/Snowy Egret (Murphy 1992, Perkins 1995, Massiah and Frost 1998).
Gularis is the west African coastal form. It is distributed from southwest Mauritania, Senegal, Mali, Gambia, Guinea-Bissau west to Nigeria, Cameroon, Gabon and Equatorial Guinea, as well as Bioko, Sao Tome, Principe and Annabon islands, occurring principally along the coasts but also inland in some areas (Walsh 1987).
Dimorpha is the Madagascar and East African form. It is occurs on Madagascar, Aldabra, Cosmoledo, and Astove Island in the Seychelles, and on Kisite (southeast Kenya), and Pemba, Zanzibar, and Mafia Islands (Tanzania) (Turner pers. comm., Baker and Baker in prep.).
Schistacea is the northeastern Africa and Indian form. It breeds along the coast of the Gulf of Suez, Red Sea, Indian Ocean, Persian Gulf, and Arabian Sea in Egypt, Sudan, Eritrea, Dijbouti, northern Somalia, Yemen, Saudi Arabia, Bahrain, Oman, Quatar, Kuwait, Iran, Pakistan, India (mainland west coast), Sri Lanka. It also has been shown to breed inland at Lake Turkana, records supported by both observations and specimens (Owre 1967, Hancock and Kushlan 1984). Breeding as far south as Somalia, there is a gap in reef heron distribution to the start of the range of dimorpha in Kenya. Birds of schistacea have been introduced to zoos and parks in Austria and Germany by Pakistani dealers Birds attributed to this introduction have been sighted in Europe (Wüst 1983). Dark morph birds nest in Israel (Ashkenazi 1993) and the subspecific identity of these and other dark birds in Europe remains unclear.
Nigripes is the Indonesian form. It nests in Indonesia (Sumatra, Java, Borneo, Lesser Sunda, Mollucas, Palau islands).
Immaculata is the Australian form. It breeds in northern and eastern Australia.
Nonbreeding range: Garzetta occurs in the nonbreeding season widely in south Europe (Spain, Portugal, south and west France, Italy, and recently England) (Barbieri and Fasola 1986, Biondi et al. 1994, Hafner et al. in press), Middle East (Azerbiajan, Iraq, Iran), sub-Saharan Africa, Southeast Asia, east Asia (Japan, south west China). Philippines, New Guinea, and Australia. Some Australian birds move for nonbreeding season to Tasmania, New Zealand, New Guinea, New Britain, and Philippines. Other populations spend nonbreeding season in their breeding range.
Migration: Northern populations are partially migratory, and both northern and coastal populations are highly dispersive. Tropical populations appear to be non-migratory.
In northern breeding populations of garzetta, dispersal occurs in July–September, migration occurs in late August or September, returning in March–April. Numbers wintering in Europe have increased since the 1950's. In fall, European birds move to south to and through France and Spain to central and west Africa. Birds from west Spain move south to the south of the Iberian Peninsula to north Africa, whereas birds from east Spain disperse to the Mediterranean and then migrate to west Africa (Bartolome et al. 1996). European birds also move south to and through Italy to north Africa, some continuing a transSaharan migration to central and west Africa, where they winter in Senegambia, Mali, Guinea, Sierra Leone, Ghana, Nigeria, Cameroon, and Sudan. Birds for further east in Europe move south and west to and through Israel, Iraq, and Iran, some wintering there others moving down the Red Sea coast to Eritrea, Djibouti, Somalia, and East Africa (Ashkenazai 1993, Turner pers. comm.).
East Asia birds move to south China, Southeast Asia, and Philippines (Anonymous 1987). Japanese birds are partially migratory, with much of the breeding population remaining year round. Other birds move to the Philippines. Taiwan birds also move to the Philippines.
Post breeding dispersal and spring migration overshoots are common, bringing birds to outside their breeding range. Dispersion records include central Russia, China (Hopeh, Shantung, Knasu), Korea, Cocos-Keeling Island, Norfolk Island, Christmas Island, Hawaii, Germany, Poland, Switzerland, Belgium, Denmark, Norway, Sweden, Netherlands, England, Ireland, Iceland, Faroe Islands, Madeira, Canary islands, Azores, Newfoundland, Martinique, Puerto Rico, east United States, St. Lucia, Trinidad, Tobago, Guyana, Suriname (Haverschmidt 1983, Forster 1989, Holmstrom 1989b, Combridge and Parr 1992, Olafsson et al. 1992, Perkins 1992, Pratt and Bruner 1992, Woods 1994, Ryan 1997, Hayes and White in press).
Gularis may be partially migratory, although evidence remains sparse. Birds occurring in Gabon, where breeding has not been recorded, may be migrants or wanderers from breeding colonies on nearby Sao Tome and Principe Islands. However, this race certainly does disperse widely, as shown by records from the Cape Verde Islands, Azores, Spain, south France, Sicily, Hungary, Bulgaria, and east United States, Puerto Rico, St. Lucia, and Barbados, Trinidad, Tobago (Cordillo et al. 1983, Smith and Hutt 1984, Murphy and Nanan 1987, Georgiewa 1999, Butler et al. 2000, Kenefick and Hayes 2001).
Northern populations of immaculata seem to be sedentary. Eastern birds disperse in nonbreeding season to Tasmania, New Zealand, and New Guinea. Nigripes, schistacea, and dimorpha also appear to be non-migratory. Dispersal records of dimorpha exist from Comoros, Seychelles, and Reunion islands.
Status: Populations of garzetta were marked by declines and population contractions due to hunting in the 19th century. This was followed by a recovery that has now achieved expansion well beyond its historic range. Overall, it is common to abundant within its range in Europe, Africa, and Asia, and widespread although not common in Australia and now has a foothold in the Western Hemisphere.
In Europe, it is expanding its range to north France and the British Isles and populations have increased markedly in France, Italy, Spain, and Portugal. The total European population is now about 47,000-70,000 pairs (Marion et al. 2000). The largest European concentration of breeding Little Egrets is now in Italy. Also in Europe, its range is increasing with first nesting in Netherlands in 1979, United Kingdom and Ireland in 1998 (Voslamber 1992, Lock and Cook 1998, Anonymous 1998, Smiddy and O’Sullivan 1998). In Netherlands, it bred intermittently for several decades, 1979, 1992, 1994-1996, and 2000. In the United Kingdom, the breeding population is increasing quickly, 2 pairs in 1996, 7 in 1997, 15-16 in 1998, 18 in 1999 (Hafner et al. in press). The egret was eliminated from the Volga Delta (Russia) in the early part of the century but is now stable at 3,000-4,000 pairs.
In Africa, breeding populations along the Mediterranean are few and scattered. About 40 pairs breed in Algeria; it is decreasing in Morocco with sites being abandoned; there are several small colonies in Tunisia and several hundred pairs in Egypt (Hafner et al. in press.). A total of 1897 nests of gularis were counted on Banc d’Arguin, Mauritania, in May December 1997 (Hafner et al. 1988). In Senegal several thousand birds breed in the Saloum Delta. In Mali, up to 1,000 pairs of garzetta and 90-110 pairs of gularis breed in mixed-species heronries in the Inner Niger Delta. Population estimates for Tanzania are as many as 15,000-20,000 coastal birds (ascribed to dimorpha); garzetta is surprisingly common around Lake Victoria, where over 25,000 have been calculated to occur seasonally (Baker and Baker in prep.) Elsewhere, dimorpha is common throughout Madagascar, with up to 400 pairs breeding regularly in a mixed heronry in Antananarivo.
In the Middle East, populations have increased in Israel to 2,000 pairs. There are up to 8,000 pairs in Azerbaijan, 100 in Bahrain, 920 pairs in Iran, and 110-120 in Oman. In Asia, it is common in India, Thailand, Japan, Java (1,000 pairs at Palau Dua), and China (at two sample areas: 2,000 nests in 10 heronries near Lake Poyang and 9,700 nests in 5 heronries near Lake Tai) (Fasola pers. comm.). In Australia, its overall range has expanded during last century, being recorded first in South Australia in 1952, Tasmania in 1957, Western Australia in 1965, and New Zealand in 1951 (Maddock 2000).
In America, the Barbados population was most recently 40 birds (Butler et al. 2000). Little Egret sightings are increasing in elsewhere in the West Indies, such as in Trinidad and Tobago (Hayes and White 2001).
Habitat
The Little Egret typically feeds in open or sparsely vegetated shallow to very shallow water using a wide variety of habitats, both inland and coastal, such as the banks of gently flowing rivers and streams, shallow lakes, pools, lagoons, irrigation canals, flooded meadows, open areas in swamps (Melaleuca, Eucalyptus, mangroves) and marshes (Typha, Phragmites, Scirpus), coastal mud flats, sandy beaches, rocky shores, coral reefs, mangrove covered shores, tidal streams. Use of artificial habitats is very typical, especially rice fields, fish ponds, and irrigation pools. It feeds frequently from floating vegetation. In Lake Victoria, East Africa, they use floating hyacinth (Eichhornia), and may be seen in the hundreds flying about searching for unoccupied rafts to claim (Baker and Baker in prep.). Terrestrial habitats are not avoided, where they feed commensally with cattle or other ungulates. It is generally a lowland species but is found up to 1,400 m in Nepal, 1,740 m in New Guinea, and 2,000 m in Armenia.
Protected trees, bushes or islands are needed for nesting. In such sites they may nest in many situations. For feeding, studies contrasting artificial and natural breeding sites have been able to delineate microhabitat preferences (Hafner and Britton 1983, Hafner et al. 1986, 1993, Fasola and Ghidini 1984, Fasola 1986). Most typically the Little Egret feeds in shallow (10-15 cm deep), open and unvegetated sites where water levels and dissolved oxygen are fluctuating (tidally, seasonally, or daily) where fish are by being concentrated in pools or at the water’s surface. Egrets will switch habitats through the year, in one study from freshwater marshes to salt marshes to rice fields for breeding (Kazantzidis and Gouter 1996).
Foraging
The foraging behavior, food, and feeding ecology of the Little Egret has been well studied (Fraser 1974, Clancey 1976, Hafner 1977, Balanca 1987,Voisin 1978, Sueur 1979, Hafner et al. 1982, Fasola et al. 1981, Fasola 1982, Fasola and Ghidini 1983, Hafner and Britton 1983, Davis 1985b, Balanca 1987, Britton 1993, Fasola and Ghidini 1983, Kersten et al. 1991, Berezokikov and Gistov 1994, Zhang et al. 1994).
The Little Egret feeds almost exclusively by day, when it is able to search for its prey visually. Conditions that disrupt water clarity affect feeding (Cezilly 1992). The bird’s usual feeding technique is to Walk Slowly through shallow, open water searching for fish or other prey. It periodically stops walking to Stand and examine a potential prey item. It also may Stands for longer periods in the water, on the shore or on a floating raft of vegetation. It intersperses Walking with more active behaviors such as Walking Quickly, Running after prey, Foot Stirring, Open Wing Feeding, Double Wing Feeding (Demey 1986, Voisin 1991, Phalan 1997). Over deeper water it can feed by flying - Dipping, Foot Dragging, Jumping, and Hopping from one spot to another (Hirschfeld 1991).
In its usual Walking foraging, the neck may be stretched out in any position from vertical to horizontal. It walks slowly with frequent halts to Stand and wait for a prey item to show itself. Foot Stirring in the mud or sand bottom or submerged plants is used to inspire its prey to move. If no prey is seen after Foot Stirring, the egret may stir again or move on. Egrets pursue swimming fish with Open Wing feeding, Running rapidly back and forth, and Hopping from place to place. At the end of a foraging sequence, the bird will either stab at a prey or stopping to look more carefully. In rice fields, Walking accounted for 62% of foraging time, Walking plus Foot Stirring 32%, Standing 6%; whereas in streams Walking consumed 82% of the time, and Walking plus Foot Stirring accounted for the remaining 18% (Fasola 1994). Types of prey account for much of the variability in the foraging techniques used. Egrets catch large, mobile fish by Standing; medium, mobile prey by Walking and chasing; and small, and nonmobile prey by Walking (Yamada 1994). The catch rate of Little Egrets is high relative to other herons (Voisin 1978).
Little Egrets feed both solitarily and in aggregations. Solitary feeding occurs along the coast and inland, typically in situations where prey is not concentrated. Gularis is considered to be characteristically a solitary feeder (although it does feed in aggregation as well) (Bundy 1985).
At sites of high food availability, Little Egrets feed by joining other birds to form single and mixed species aggregations. This may occur only in the morning when low oxygen forces fish or prawns to the surface, after which they return to solitary foraging (Kersten et al. 1991, Hafner et al.1993). Striking efficiency and capture rates are higher when birds are feeding in aggregations than alone (Hafner et al. 1982, Cezilly et al. 1990). Juveniles are less efficient then adults when feeding alone, but efficiency becomes similar when they feed in aggregations, showing the energetic advantages of flock foraging for this species (Cezilly and Boy 1988).
When feeding in aggregations, interactions with other species are complex. Little Egrets defend feeding sites and individual distances against nearby birds, including other species notably night herons. Territoriality is strong both when feeding alone, such as along a linear stretch of beach, and even within aggregations (Chu and Wang 1988). In flock feeding, they use diverse opportunities to forage commensally, such as by following species as diverse as cormorants (Phalacrocorax), ibis (Threskiornis), spoonbills (Platalea) or even jackals (Canis). They also frequently rob other birds of prey and are subject to being robbed by a wide variety of other species (de Plessis 1986, Amat and Aquilera 1989). Other ways of feeding include using floating bread or their bills to attract fish, following cattle, and riding bathing water buffalo (Barden 1990, Parasharya and Bhat 1987, Balasubramanian 1990, Tsuboshima 1994, Wooldridge 1998, Whitehall 1999). Birds roost when not feeding, and in the evening outside of breeding use communal roosts (Itoh 1984a, b).
The Little Egret’s food is mainly small fish, generally only 1.2-6 cm long, averaging about 4 cm. Fish include introduced topminnows (Gambusia), chub (Leucisus), perch (Perca), carp (Cyprinus, Carassius), stickleback (Gasterosteus), eel (Anguilla), gobies (Gobius), tench (Tinca), loach (Cobitis), mullet (Mugil), sunfish (Eupomotis), sand-smelt (Atherina), and many more species recorded. The egrets will also eat frogs and tadpoles (Rana, Hyla, Pelobates), toads (Bombina), snakes. Insects include beetles (Dytiscus, Cybister, Hydrobia), dragonfly larvae, mole cricket (Gryllotalpa), cricket (Gryllus). Crustaceans include prawn (Palaemonetes), amphipods (Gammarus), phyllopod (Triops), introduced crayfish (Procambarus), crabs (Hymenosoma). Other invertebrates are spiders, worms, snails (Assiminea), and bivalves (Musculus). They also will eat lizards, snakes and small birds such as small chickens and warblers (Howe 1989).
Little Egrets are hugely opportunistic, taking advantage of what prey that are locally abundant or accessible. The diet is regionally variable, with fish or invertebrates being relatively more or less common in different places. The diet in natural marshes tends to be dominated by fish, whereas those feeding in rice fields tend to take invertebrates, fish, or frogs depending on availability. Coastal Little Egrets have a high proportion of crustaceans and mollusks, a diet that may explain the relatively thick bills of these populations.
Breeding
The breeding biology of the Little Egret has been studied throughout much of its range (Blaker 1969, Voisin 1976, 1977, Naik et al. 1981, Inoue 1985, Parasharya and Naik 1988, Zhang and Liu 1991, Prashant et al. 1994, Kazantzidis et al. 1996, Rao et al. 1996, Thomas et al. 1999).
As would be expected, the breeding season varies across its large range. In the northern parts, the Little Egret nests in spring and summer, March–July in Europe and north Asia. In tropical areas, breeding generally coincides with periods of high rainfall, most often peaking with or immediately following the height of the rains. Main laying dates vary considerably throughout Africa (Turner pers. comm.). They are generally April–August in India, June–August in Vietnam, May–June in Borneo, January–April in north Australia, November–January in south Australia.
Little Egrets nest in many substrates, including trees up to 20m high, bushes, reeds on ledges or rocks. It will nest on the ground where protected and also in areas of human habitation (Subramanya 1996, Kobayashi 2000).
The Little Egret is a highly gregarious species that nests colonially, sometimes in large mixed-species colonies of other herons (especially Black Crowned Night-Herons), ibises, and cormorants, with some colonies numbering in the thousands. Aggressive behavior in colonies can expand distance between nests to 4 m, but in dense colonies nests may be as close as 1 m apart. Coastal birds tend to nest in smaller colonies or alone. Nests are small platforms, 30-35 cm wide, 10-15 cm high, made of sticks. They are built by both sexes usually with sticks brought by male. Nests continue to be added to through incubation (Zhou et al. 1998).
Males display from a succession of temporarily established advertising stations. These are defended vigorously by Upright displays, Head Flicking, Twig Shake, and Forward displays, first by the male then by both birds of the pair. In the Forward, the egret erects its crest, neck and back plumes and gives the Ggrow call. Forwards have several intensities. Males also do Supplanting Flights.
The Stretch is the advertising display, serving to attract females. The bird crouches, puts its bill vertical, erects its back plumes, and pumps head straight up and then down, giving Ow call. It goes up and down in either slow motion or in short, rapid movements, finally returning the bill to horizontal. Also when advertising, birds give Doo or Po calls, stand Preening, and conduct Circle Flights with neck outstretched producing a loud whomping wing sound.
After pairing, Preening, Back Biting with Non Contact Bill Clappering are frequent. The Greeting Ceremony involves the approaching bird giving the Da-wah call and the attending bird giving an Upright display and also calls Da-wah. Both birds give a Forward display with plumes and crest erected, and Bill Clapper.
The eggs are variable green-blue, fading to an off-white. Measurements vary regionally. Eggs from South Africa average 46.5 x 34.2 mm, from Sri Lanka, 42 x 33 mm, from China 44.3 x 32.3 (Zhang et al. 1994). Clutch size varies geographically, within a range of 2-8. Clutches average 4.9 in Japan, 4.8 in Spain, 2.4-2.9 in Senegal, 2.6 in East and southern Africa, 2.5 in Madagascar, and 2.2 in Australia.
Both sexes incubate the eggs, starting with the first egg and providing full incubation after the second or third egg. Incubation period is 21-25 days. Chicks hatch asynchronously. The young are semialtricial and nidicolous. Both parents attend them during the guardian period of 10-15 days. However in some situations one of the parents may abandon the nest after the guarding stage, possibly in order to renest (Fujioka 1989). Chicks are fed by regurgitation, first onto the bottom of the nest and after a few days directly from the parent’s bill (Inoue 1985).
Growth is rapid. Nestlings compete for food, with the older nestlings being more successful. The result of this competition is usually a brood reduction (Inoue 1985). Mortality may be high owing to starvation (Voisin 1976, 1977). Young leave the nest at 35-50 days, variable among colonies.
Hatching success is high, 90.2% in Israel (Ashkenazi and Yom 1997), although egg mortality occurs in large clutches possibly due to limits on the number of eggs that can be effectively incubated (Fasola 1998). Chick survival is influenced by predation, weather, rainfall and hydrology, and prey availability (Baxter 1994, Maddock 1986b, Bellinato and Bogliani 1995, Hafner et al. 1986). Of these prey availability is usually the most critical. Birds have the greatest nesting success in years having high fish densities in natural marshes or high prawn densities in rice fields (Hafner et al. 1986). A primary cause of brood reduction is nestling mortality due to competitive aggression by siblings. In Australia 1.46 young fledged per nest in dry years and 2.59 for nests in wet years, when food was more available (Maddock 1986a). Similarly, a colony site from which egrets fed on prey concentrated in early morning by low oxygen had higher production (3.25 chicks per nest) than colonies without such prey vulnerability (2.69 chicks/nest) (Hafner et al. 1993). Predators attack chicks in the nesting colony. These include hawks (Accipiter, Circus), owl (Bubo), and crows (Corvus) (Kayser 1995, 1996).
After fledging, young will frequently pursue the adults flying after them through the colony uttering load and raucous Ka calls; the parents will land in a tree to feed them (M. Maddock pers. comm.).
Population dynamics
A substantial proportion of Little Egrets appear to breed in their first year, unusually precocious for herons (Hafner et al. 1998). Adult and juvenile survival, rather than nesting success, is the most critical demographic parameter (Hafner et al. 1999, Hafner et al. in press). The average number of eggs in a clutch size appears to be fewer than would be optimal for the production of the most young (Fasola 1998). This finding suggests that it may be more important to reduce stress on the adult than to maximize production of young in any one year. Survival of young varied in the first year from 6.5 to 55.2 % (Hafner et al. 1998). Hatch order of juvenile, the effects of mild winter weather, and food supplies immediately post fledging did not influence survival. Adult survival is a constant 71.4%. Mortality factors include winter kills, migration risks, and predation (Hafner et al. 1992, Combridge 2000). Hard winters in which feeding sites freeze over can kill wintering birds (Hafner et al. 1994). It is likely that the long period of successive mild winters has contributed to the strong range expansion in Europe (Hafner et al. in press), and by extension its dispersal and colonization of other areas.
In France, reproductive parameters, such as clutch size, have decreased over time corresponding to increases in population (Bennetts et al. 2000). This finding suggests that reproductive parameters are in part density dependent. Another important aspect of the population biology is the species’ ability to shift among nesting sites, even over thousands of kilometers in response to adverse conditions (Hafner et al. in press). Thus local population losses (such as through a hard winter) can be compensated by inflow from other areas.
Conservation
Human populations are large and dense in the range of the Little Egret. Fortunately, the species is tolerant of human presence except where persecuted. In India, 53% of colony sites were in or close to human habitation (Subramanya 1996). Colony sites must be safe from terrestrial predators and secure from direct human disturbance. Consequently, a critical conservation action is to expand surveys range-wide to identify, catalogue, preserve, protect, and manage important colony sites. To protect the species across its range, it is necessary to preserve a network of active and potential colony sites, and given the destruction of natural sites that has occurred, create artificial nesting sites in areas where feeding conditions are adequate but no suitable site exists. Natural wetlands are the traditional foraging habitat of the species. Loss of inland and coastal wetlands has occurred throughout its range, especially in south Europe, north Africa, Middle East, Iran-Iraq, and India. This has resulted in the large-scale destruction of heron habitat. Threats remain in all areas, especially along the Mediterranean and Black seas, where some of the largest colonies occur. Remaining natural habitats need to be actively managed for prey and protected from further habitat degradation (Kazantzidis and Goutner 1996). Especially important are the few large wetlands that, over the past decades, have been critical to the species (Van Dijk and Ledant 1983, Pyrovetsi and Crivelli 1988, Puglisi et al. 1995a, Prosper and Hafner 1996). Some of these, such as the Mesopotamian Marshes and the Black Sea Lowlands, are under immediate threat.
Throughout the Little Egret’s range, wetlands already have been drained for agriculture, including for rice farming and fish farming. Agricultural and industrial operations influence contaminant burdens of egrets nesting nearby (Berny et al. 2002). Nonetheless, rice fields and fish farms are now important feeding habitats for Little Egrets throughout their range. Rice fields have become exceptionally important feeding areas in some regions such as the Ebro Delta and Albufera de Valencia in Spain, the Po Valley in north Italy, the Axios Delta in Greece, and the Goksu and Cukurova Deltas in south Turkey (Fasola and Barbieri 1978, Voisin 1978, Fasola and Ghidini 1983, Biondi et al. 1993, Fasola and Ruiz 1996, 1997). Rice fields support 39% of the Italian Little Egret population, now the largest in Europe. The Development of rice farming explains a large part of the species’ population increase there (Fasola 1983, 1986). An excellent study of these colonies (Fasola and Alieri 1992b) demonstrated the strategy for conserving Little Egrets in developed areas: protection of a network of colony sites from disturbance and ground predators, identification of feeding areas, active management of identified feeding habitat to retain critical characteristics, and establishment of new colony sites in appropriate feeding habitat currently lacking nesting habitat. Changes in rice farming practices around the world raise concern as to the immediate and long-term effects on Little Egrets and other herons (Narusue and Uchida 1993, Lane and Fujioka 1998). Agricultural policy should be developed to protect and continue rice culture techniques of value to egrets. Fish ponds are another artificial resource used by Little Egrets across its range. Interactions of egrets with aquacultural facilities are a conservation challenge. Studies have suggested that there are both costs and benefits to aquaculturalists from egret predation (Ashkenazi and Yom-Tov 1996). In some areas the egrets have become dependent on these ponds (Young and Chan 1997). The loss of fish pond habitat in Hong Kong has caused a decline in the nesting Little Egret population there (Young 1998). Methods need to be found to preserve access to fish ponds where they are essential to population health.
Research needs
The Little Egret, thanks in large part to the work of Heinz Hafner, Mauro Fasola, and their colleagues, is now one of the best-known herons. Its breeding biology and feeding ecology are well understood and provide insight into other species. It also is one of the few species in which demography is at least partially understood, demonstrating the value of long-term banding studies. An expansion of similar studies into other parts of the species’ range is highly desirable. A principal research need is to definitively unravel patterns of geographic variation and the taxonomic identity of the populations presently attributed to this species. As discussed above, much still needs to be discovered to fully assess the hypothesis that these populations represent a single polymorphic species. At the core of their ranges Little Egrets in Europe and Reef Herons elsewhere do differ on average in bill shape, body shape, and habitat. However, when light and dark birds come together, such identities become less discernable because individual variability blurs characters that might appear distinctive comparing typical forms. Studies have yet to document whether the Little Egret of the European and Asian mainland is dimorphic, seemingly an important issue. Furthermore, these are birds that disperse widely and shift populations in response to changes in habitat conditions, leading for example to coastal egrets starting to breed inland in India. Thus, some interbreeding may be relatively new, which in itself says little about relationships of the forms other than they are close. It is this variability, uncertainty, and repeated inability to tell specimens apart that suggests the hypothesis of conspecificity. Testing the hypothesis requires range-wide studies of morphological, behavioural, and genetic/biochemical characters. It is as likely that there is one species as it is that there are four or more species in this group.
Overview
The Little Egret is a species characterized by its flexibility, which permits a diversity of ecological strategies across its large range. Among populations currently identified as Little Egrets there is considerable variation in bill, leg, and plumage, some of which have ecological implications. Coastal populations tend to have shorter legs and stockier bills, probably to feed on harder prey. In dimorphic populations, the ratios of colour forms differ geographically, probably as a response to slightly differing selective pressures in different habitat conditions. Populations may be sedentary, migratory or partially migratory, and the proportion of a population migrating changes with time. This behavioural diversity likely balances migration vs. winter risks over time. Individuals move around and populations shift in response to local conditions, for example shifting from Spain to France due to drought. The tendency of individuals to disperse results in their colonizing distant places, including a recent invasion of the New World.
The Little Egret is able to adapt its foraging to the situation at hand. It has a wide repertoire of behaviours, some of which are used in exceptional circumstances, but, the vast majority of time it uses only a few behaviours, Walking slowly with Foot Stirring, Standing and Running, each used to capture different sizes and types of prey. By targeting particular prey types, it has a high prey capture rate, higher than any other European heron. The egrets’ opportunistic yet efficient approach to foraging results in a high food intake, despite small prey size, and a resulting high reproductive output. Versatility, high reproductive capacity, early age of first nesting, high adult survival, diverse winter strategies, and dispersive tendencies resulted in the species being able to increase its populations and expand its range so remarkably over the past century.