Rufous Night-Heron
Nycticorax caledonicus (Gmelin)
Ardea caledonica Gmelin, 1789. Syst. Nat. 1 (2), p. 626: New Caledonia.
Subspecies: Nycticorax caledonicus manillensis Vigors, 1831: Manila (Philippines); Nycticorax caledonicus mandibularis Ogilvie-Grant, 1888: Aola, Guadalcanal (Solomons); Nycticorax caledonicus hilli Mathews, 1912: Parry’s Creek, NW Australia; Nycticorax caledonicus pelewensis Matthews, 1926: Palau Islands.
Other names: Nankeen Night Heron in English; Martinete Caneto in Spanish; Bihoreau cannelle in French; Rotrückenreiher in German; Hashibuto-goi in Japanese; Lapay degar in Tagalog (Philippines); Baku gabi in Pilipino; Koward merah in Indonesian.
Description
The Rufous Night-Heron is a stocky rufous and white heron with a black crown and white head plumes when breeding.
Adult: The crown and nape are grey black. A line surrounding the eye may be white or rufous. The face is white with rufous wash. The short, heavy bill is black. A facial mask consisting of the lores and skin at the base of lower bill is green yellow. The irises also are yellow. The neck is short and thick. The hind neck, back, tail, and upper wing vary from light to dark - pale cinnamon, cinnamon rufous, chestnut brown, maroon. The underparts are white, with rufous or similar wash. The under wing is white, with cinnamon rufous flight feathers. The neck and upper breast often have a pale chestnut wash, with the white of the undersides extending over the front of the wing. The short, thick legs and the feet are green, cream yellow, to orange yellow.
In breeding plumage two or three long white plumes emerge from the crown down the back. When fresh, the plumes are tipped with black. In courtship lores turn blue; irises are red turning orange; legs turn bright pink or red.
Variation: Sexes are alike but females average smaller in most measurements. Five subspecies are based primarily on size and back plumage color. In addition there is much color variation that is not geographically based, so that even in a small area one can see various shades of colorations. Caledonicus is dull rufous chestnut above with light rufous belly. Manillensis is relatively large and dark, having dark maroon upper parts and chestnut washed under parts; the eye circle is lacking or poorly developed; and crown plumes are smoky black or white with dusky base and tips. Within the Philippines, southern birds are larger than northern birds and may have a paler base of the bill; they have been described as a different subspecies, major (Hubbard 1976). Hilli is chestnut brown above white on the belly. Pelewensis is dull maroon. Mandibularis is relatively smaller, with rich chestnut upper parts, the color extending around the neck and chest; it lacks the eye circle; and it can have crown plumes with a black wash. Birds of New Britain and new Hanover Islands have been described as intermediate between hilli and mandibularis (Amadon 1942). Birds intermediate between hilli and manillensis are described from Java and Borneo (Amadon 1942, Hoogerwerf 1966).
Juvenile: The immature is rufous brown with much buff and white spotting and streaking. The crown is black brown with buff streaking. Iris is yellow to orange yellow and the facial mask of lores and next to lower bill is yellow olive, developing a blue tint. Upper bill is grey black with yellow edge whereas the lower bill is yellow olive with grey black edge and tip. The chin and upper throat is white. Neck is buff and brown streaked. Back is rufous brown with buff spots. The rump is white but the upper tail is brown with large buff spots at the tips of the feathers. The tail is rufous brown with dark brown barring and narrow white feather tips. The upper wing is brown rufous with large buff spots at the end of each feather. Under wing is white with grey brown steaks and flight feathers are rufous brown with dark brown inner edges. The under parts are brown and cream streaked. Legs and feet are lime green to olive grey. With age the crown becomes blacker and upper parts more rufous and less spotted.
Chick: The chick is covered with dark brown down on the back and white on the under sides. Bill is cream with grey black edge. Iris is orange yellow to yellow and the lores are white to orange pink. Legs and feet are olive.
Voice: The characteristic “Quok” call is the flight call. The “Rok” call is the threat call, used in feeding aggregations.
Weights and measurements: Length: 55-65 cm. Weight: 810-1,014 g.
Field characters
The Rufous Night-Heron is identified by its back cap and chestnut to brown back. The adult is distinguished from the Black Crowned Night-Heron by its rufous (not black) upper parts.
The immature is similar to other cryptic herons. It is distinguished from the adult by its larger size, its light underparts, uniform rufous upper parts, yellow legs, and gregarious habits. It is distinguished, only with difficulty, from the immature Black Crowned Night-Heron by its rufous brown (not grey brown) appearance, flight feathers rufous brown with dark brown inner edges (not grey brown), dark brown buff spotted upper tail covers (not brown), white (not grey brown) rump, tail rufous brown with dark brown barring (not grey), and slightly smaller size, on average. It is distinguished from the Australasian Bittern by its shorter neck, hunched posture, rufous (not brown) upper parts, spotted (not barred) wings, and less dense habitat. It is distinguished from the juvenile Striated Heron by its larger size, light spotting on back and wings, and pale under parts.
Systematics
The Rufous Night-Heron is closely related to the Black Crowned Night-Heron and these species have hybridized in Java and Borneo where their ranges have come into contact. They overlap in a minor way in the Philippines and in the past in Sulawesi (Hoogewerf 1966, Hubbard 1976, Erftmeijer 1989). There is not evidence of persistent present day hybridization (Sheldon and Marin 1984). It is likely that the ranges have come into overlap due to relatively recent invasions of Indonesian islands by the Rufous Night-Heron and of the Philippines by Black Crowned Night-Herons.
The two birds are nonetheless mostly allopatric. The Rufous Night-Heron has been in the Philippines and islands to the southeast long enough to have diverged in size and color. As far as is known, the Black Crown Night-Heron has not racially differentiated in the area, suggesting that it is the more recent invader. It would appear that attempts by the Rufous Night-Heron to colonize other islands in the past century have had limited success. Given the intermittent history of range overlap, and nonpersistence of hybrids, it seems likely that these are two good species.
Range and status
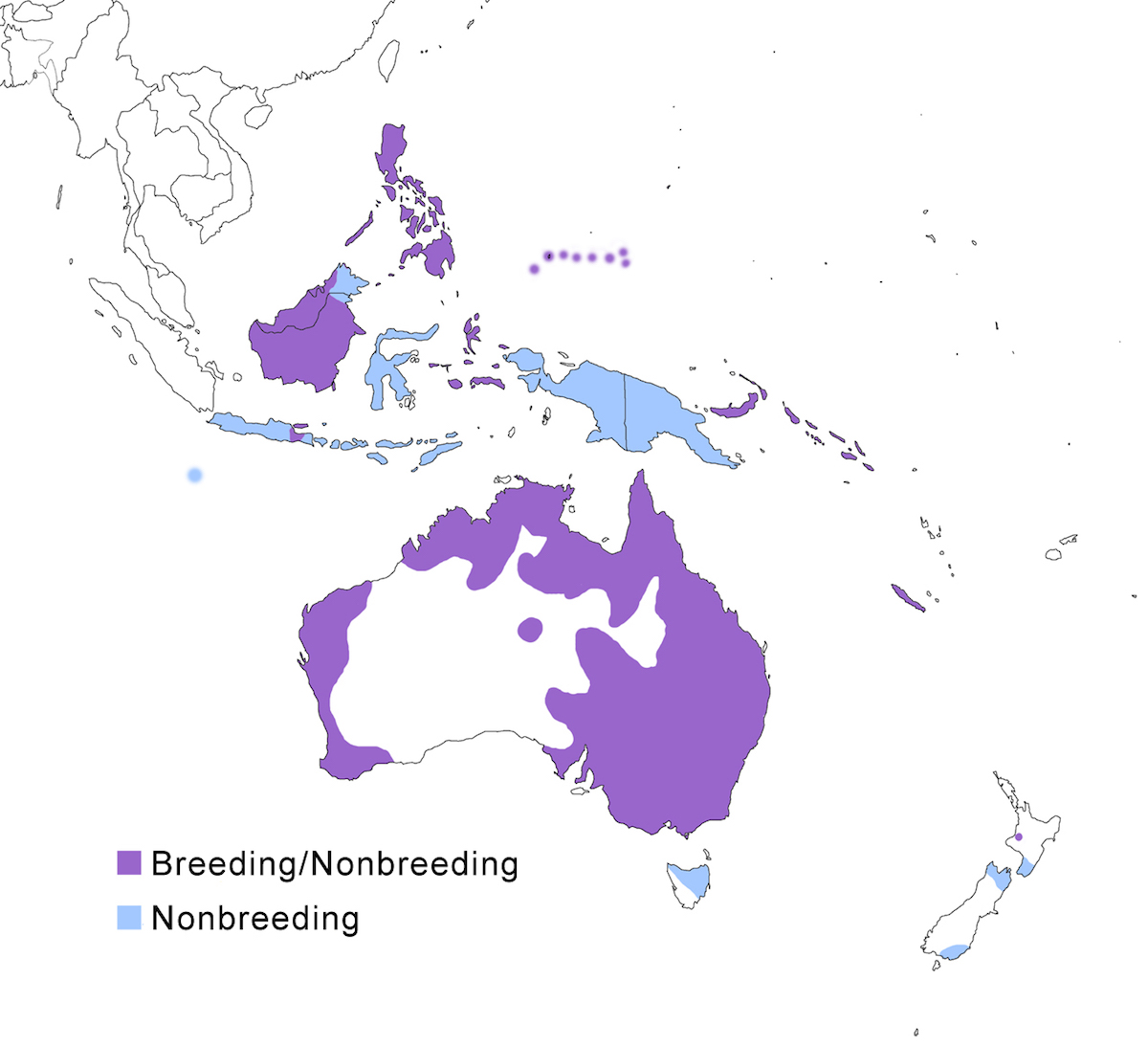
The Rufous Night-Heron occurs in Indonesia (Java, Borneo, Celebes, Lesser Sundas), New Guinea, the Philippines, Palau, Micronesia, New Caledonia, Bismarck Archipelago, Solomon Islands, Australia and New Zealand.
Breeding range: Caledonicus occurs in New Caledonia. Manillensis occurs throughout the Philippines (Luzon, Mindanao, Marinduque, Negros, Panay, Palawan, Sulu Archipelago), west Indonesia, and north Borneo (Hubbard 1976). Pelewensis occurs on Palau and Micronesia. Mandibularis is found in the east Bismarck Archipelago, New Britain and the Solomons. Cassirostris previously occurred on the Bonin Islands (Japan).
Hilli breeds in Australia and in very small numbers in recent years in Java (MacKinnon 1993, Erftemeijer 1989), Borneo (Sheldon and Marin 1984), and New Zealand (Marsh and Lövel 1997). In Australia it occurs in north, east, and south west Australia. Nesting populations are concentrated in the south east in Queensland, New South Wales, and Victoria, but records indicate wide spread nesting throughout its Australian range including the Great Barrier Reef. Introduction into New Zealand in the mid 1800’s failed (Heather and Robertson 1996). It may have bred in 1957-9 and was discovered nesting in 1995 (Marsh and Lövel 1997). It also has nested in the past on Celebes and Cocos-Keeling, but recent records of nesting are not available.
Nonbreeding range: Hilli occurs in the nonbreeding season in New Guinea, west Bismarck Archipelago, Lesser Sundas Islands, New Zealand, and Cocos-Keeling.
Migration: The Rufous Night-Heron is partially migratory in the south of its range. Birds move north in the Austral winter as far as Papua-New Guinea. Depending on the persistence of suitable feeding and nesting conditions, some populations are sedentary, others more nomadic, moving around response to rainfall and flooding events to take advantage of newly opened foraging conditions. In Australia, birds move into and out of the Darling-Murray River system depending on water conditions, large colonies forming in wet years.
Substantial post breeding dispersal of young takes place, which gives rise to quite long distance wanderings. Australian birds disperse (or migrate) regularly to New Guinea. Dispersal birds also occur in Tasmania, New Zealand, Lord Howe Island, Christmas Island, and Cocos-Keeling Island. This is probably how the species colonized so many islands.
Status: Hilli is widespread, common, and stable in Australia in the north, east and southwest, but rare or absent from the interior and northwest Western Australia. It is common to abundant in nonbreeding season in New Guinea, numbers fluctuating from year to year. The breeding population is very small on Java. It breeds regularly throughout north Borneo (Mann 1989, Lansdown et al. 2000). It is rare in Celebes and Lesser Sundas (Mason 1994). Caledonicus is common in New Caledonia. It is one of the most common birds on Cocos-Keeling (Stokes et al. 1984). Cassirostris, discovered on the Bonin Islands in 1820, became extinct by 1889.
Habitat
The Rufous Night-Heron feeds in a variety of habitats, mostly shallow with still or slow moving water and tall but not overly dense vegetation that offers some degree of cover. It is usually on the margins of deeper water or in flats and open areas within marshes, swamps, and forests. Their habitat ranges from the coast to inland freshwater, from permanent water bodies to temporary pools, and from swamps to dry grasslands. It most abundantly uses the varied habitats of river floodplains.
Among specific habitats used by these night herons are wooded swamps, creeks, pools, lake and river margins, reed swamps, paper bark forest, eucalyptus forest, damp meadows, and dry grassland. Birds occur less often in saline areas but they are found on offshore islands, exposed rocky shores, reefs, beaches, estuaries, mangroves swamps, salt marshes, and shallow lagoons. They readily use human situations including flooded pasture, dry fields, urban wetlands, ponds, parks, gardens, airports, road edges, grain bins, and garbage dumps. Most of its habitat is near sea level but it is found to 1,600 m in New Guinea.
Being nocturnal, it requires day resting spots. It roosts in dense vegetation, such as leafy trees, dense bushes, reeds, and other waterside situations, such as a log jam (Marchant and Higgins 1990). It has been seen to use dead trees, but such situations are generally too exposed. In coastal areas it uses dense low shrubs where available. They choose roosts in developed or even urban areas using introduced trees such as cypress and pines.
Foraging
The Rufous Night-Heron is nocturnal bird, feeding primarily at night and in the morning and evening. Its typical nocturnal foraging behavior is Standing and Walking Slowly in shallow water (Recher and Holmes 1982, Recher et al. 1983). It Stands in Crouched or Erect posture peering into the water looking for prey. It also will Walk slowly through the water searching for prey.
Night-Herons often are solitary foragers, maintaining exclusive well defended feeding territories. They also are gregarious for much of the year, when prey is concentrated or habitat limiting, assembling into feeding groups of a few to several hundred birds.
Although a nocturnal species, it feeds during the day during breeding in order to increase food available to young. During the day it can use more active behaviors, especially for feeding in deep water such as Feet First Diving and Swimming Feeding (Loyn 1985). It also is an opportunistic feeder taking advantage of food resources such as garbage dumps, mouse plagues, and sea turtle nesting.
Except during breeding it usually roosts during the day, in thick foliage. Roosts are usually communal, with a few to hundreds of birds using the same place. They roost singly but more often in large groups, flying from and to roosts at after dusk and before dawn giving the Quok call. Roosts are located convenient to feeding grounds and use varies with feeding conditions, some being used for many years.
The Night-Heron typically eats crustaceans, insects, fish and frogs. Crayfish (Cherax, Euastacus) appear to be a dominant prey in many situations. It also frequently eats shrimp and crabs (Holthuisiana). Fish taken include introduced mosquitofish (Gambusia) and carp (Carassius). Insects include water beetles and larvae, crickets, water bugs, caterpillars, wasps, ants (Camponotus), dragonfly larvae, termites. Frogs and tadpoles, mice (the introduced Mus), and hatchling sea turtles are important in the diet in some situations. It is a common predator on small and hatchling birds especially colonial birds, cormorants (Phalacrocorax), ibis (Threskiornis), and spoonbill (Platalea), and also non colonial species such as starling (Sturnus), ducks (Anas), and house sparrow (Passer). It also feeds on refuse at garbage dumps.
Breeding
In Australia the breeding season is variable depending on rainfall and water conditions. The usual breeding season is October–March in Australia, February–May in Philippines, June in the Philippines, and February–June in Java. Nesting on the Great Barrier reef in December–July coincides with the hatching of sea turtles. Given the development of appropriate feeding conditions, nesting can occur anytime in the year. In such colonies nesting is not synchronized (Braithwaite and Clayton 1976).
It typically nests in swamps and marshes on islands or islands of vegetation in the water. It most commonly nests in dense cover in trees, up to 20 m high, or large bushes. Along the coast, it nests in mangroves. On offshore islands, it nests on the ground adjacent within short scrubby vegetation, on rocky shores, or even under rock ledges and in caves. The night heron nests in urban and suburban settings and is especially attracted to urban parks and zoos (Melbourne, Adelaide). In developed areas, it nests in tall introduced trees, particularly pines and cypress.
The Rufous Night-Heron is a highly gregarious colonial nester in single or mixed species colonies of 200-3,000 birds, including other herons, spoonbills (Platalea), cormorants (Phalacrocorax), and ibis (Threskiornis). Within large colonies, it typically nests in separate groups, with many nests per tree, typically high in dense parts of trees. It occasionally nests alone.
The nest platform is a small, loose construction of sticks that may be only 20-30 cm in diameter and 3-4 cm high. Nests on the ground on islands may be only a few sticks that keep the eggs from rolling away. Larger nests also occur, 35-50 cm wide, 8-15 cm thick. Nests are lined with leaves. Males collect the twigs, and both parents build the nest. Sticks may be stolen from other nests.
Males claim and defend territory in the nesting colony. The Forward is given with the bird standing erect and then crouches, feathers and plumes erect, points and snaps bill. Courtship display has not been described, but is likely to similar to that of the Black-crowned Night-Heron. However, this should not be assumed. Both adults defend the nest site.
The eggs are pale green blue. They average 50 x 37 mm. The clutch is usually 2-3 eggs; range is 2-5. It is normally single brooded but can lay replacement clutches and nest more than once in a year, given proper water conditions. Incubation starts with the first egg and lasts 21 days. Both parents incubate. Hatchlings are semialtricial and nidicolous. Both parents attend and care for young. Parents feed young by regurgitation, initially by putting food into their mouth and later into the nest. Young leave the nest at 14 days, returning to be fed. After 21 days, parents will feed them away from nest. They fledge in 6-7 weeks. No information is available on breeding success.
Population dynamics
Birds generally nest in third year after adult plumage develops, but are birds in juvenile plumage are able to breed within in their first year, especially under particularly favorable conditions (Braithwaite and Clayton 1976). No information is available on demography.
Conservation
The species is widespread, common, and stable in the core of its range in Australia. Colonies can be modest (hundreds of birds) and sometimes very large (several thousand birds) under exceptional water conditions. Smaller persistent colony sites are repeatedly used, and these can be subject to local conservation and management. Larger colonies, probably representing the bulk of the generational productivity, need to be protected through conservation of the large, variable wetlands in which they occur. Throughout Australia, drainage, clearing, burning, grazing, increased salinity, groundwater extraction, and flood control have modified habitat in natural fresh water wetlands. Much of the landscape used by the heron is on private lands, and these owners must be take responsibility for conservation (Corrick 1995).
Research needs
For such a widespread species, surprisingly few studies have been conducted. Its basic breeding biology, courtship behaviour, feeding behaviour, and food choice under differing conditions need to be better determined. It should not be assumed a priori that these will be the same as the Black-crowned Night-Heron. Rather it might be hypothesized that such a flexible and nomadic species requires individual and populational responses that differ from those of the Black-crowned Night-Heron. It is intriguing that despite several invasive attempts, the species has not prospered where it overlaps with the Black-crowned Night-Heron. The study of the comparative biology of these species in their zone of overlap, such as north Borneo, is needed. The range and pattern of geographic variation within the Rufous Night-Heron deserves more exhaustive study. Given both individual and geographic variation in the species and the existence of putative hybrid specimens, a range-wide study of geographic variation is called for.
Overview
The Rufous Night-Heron is a preferentially nocturnal forager that depends on Standing and Walking. Its diet is very broad as is the case for the Black-crowned Night-Heron. However, its published diet seems to have a smaller fish component. There have not been detailed studies of the feeding or breeding biology of the species, and its similarity to the Black-crowned Night-Heron should not be assumed. One aspect of its ecology that is clear is its flexibility in nesting. While some populations appear to be relatively sedentary around permanent feeding areas, large colonies develop to take advantage of exceptional water conditions. It is intriguing that, for a species characterized by delayed maturity and prolonged juvenile plumage, under these exceptional conditions juveniles nest in great numbers. This suggests that the prolonged maturation is a reflection of normal conditions, but is superseded when foraging conditions are such that immature birds can nest successfully.