Snowy Egret
Egretta thula (Molina)
Ardea thula Molina, 1782. Sagg. Stor. Nat. Chili, p. 35: Chile.
Subspecies: Egretta thula brewsteri (Thayer & Bangs), 1909: San Jose Island, Lower California.
Other names: Garceta Nívea, Chusmita, Garcita Blanca, Garza Chica in Spanish; Aigrette neigeuse in French; Garça branca pequena in Portuguese; Schmuckreiher in German.
Description
The Snowy Egret is a graceful medium white heron with black bill and legs, and yellow feet, found throughout the Americas.
Adult: As its name implies, the Snowy Egret is all white. It has a long thin black bill, grey to yellow at its base during the nonbreeding season. The lores and irises are yellow. The long, thin legs are black with contrasting yellow feet, sometimes with yellow green extending up the back of the lower legs. In breeding condition, it has luxuriant plumes. The head plumes form a short crest. The aigrette plumes of the neck are long and filamentous. The back plumes curve upwards near the tail. In courtship the lores turn red pink and the toes orange red. The lores and feet of this species flush blood red during aggressive encounters (Hancock 1999), which are frequent.
Variation: Variation in this species is slight. Males average larger than females but are alike in plumage. Birds breeding in Baja California (brewsteri) are larger with more massive bills. Other populations are considered to be larger or smaller but the overall geographic pattern remains unclear (Parsons and Master 2000).
Juvenile: The juvenile is like the adult, without plumes. Lores are green yellow to grey yellow. The bills and legs gradually darken and so may be lighter than in the adult. The bill may be yellow horn with a dark tip. The legs although dark may be lighter than the adult with green yellow extending up the back of the lower legs from the feet, which also are green yellow.
Chick: The chick is covered with white down. Bill is pink at hatching, becoming darker at the tip, either yellow grey of dark grey at the base. The bill then darkens to black or to yellow with a dark tip. The legs are yellow grey to grey black, with toes always being a lighter shade than legs turning yellow green. Irises are grey to buff grey becoming off white
Voice: This is a highly vocal heron, particularly during interactions among individuals. It has a range of aggressive calls, the “Rah” call, the complexity of which is not adequately captured in our current understanding. These calls become harsher as the intensity of the interaction increases. A high-pitched Aarg call, rendered “aargaarg” is a disturbance call. The “Aarg”call is also used during supplanting attacks. A harsh high-pitched “Aarh” call is the Advertising Call. The male’s Stretch display is accompanied by the distinctive “Wah” call, the gurgling “a, wah, wah, wah, wah” sound that characterizes a Snowy Egret colony. Bill snaps occur during Snap display.
Weights and measurements: Length: 56-66 cm. Weight 370 g.
Field characters
The Snowy Egret is identified by its long back plumes, black bill, black legs and yellow feet. It walks upright with neck slightly arched and flies strongly and buoyantly with deep wing beats, yellow feet trailing visibly behind. It is distinguished from the immature Little Blue Heron and from the white phase of the Reddish Egret by its black legs and bill and yellow feet and lores and by its active but not frantic feeding behavior. The others often have dark or dusky feathers in their white plumage, sometimes having a grey cast. The Snowy Egret is distinguished from the Cattle Egret by its longer, thinner black bill, white not buff plumes, and its slender build. It is much smaller than the Great White Egret and white phase of the Great Blue Heron.
It is similar to Old World forms that are rare in the New World. It is distinguished from the Little Egret (which is rare but increasingly observed in east United States, Lesser Antilles, north east South America and is now breeding on Barbados) by being smaller, thinner, with longer legs, yellow (rather than green) feet the color often extending higher onto the back if the lower leg, and yellow (rather than blue grey) lores. In breeding, the Snowy Egret is distinguished from the Little Egret also by orange red feet (rather than yellow orange) extending up the back of the lower leg, red pink lores (rather than orange), more elaborate head plumes (crest-like rather than two long lanceolate plumes), and longer, recurved back plumes (Massiah 1997, McLaren 1998). It is distinguished from the Chinese Egret (accidental in Aleutian Islands) by its black (rather than orange yellow) bill, black (rather than yellow green legs) and in breeding by its red pink (rather than blue) lores.
Systematics
The relationships among the medium Egretta require additional study, particularly the closeness of the relationship between the Snowy Egret and the Little Egret, which are similar in many ways including plumage and behavior (Payne and Risley 1976, Kushlan 1976a, Murphy 1992, Vanden Berge 1970, F. Hayes pers. comm.). Despite their breeding ranges now overlap in the New Word, in Barbados, they appear to interbreed rarely if at all suggesting that they are reproductively isolated (F. Hayes pers. comm.).
Patterns of subspecific geographic variation are unclear (Parsons and Master 2000). While the birds of Baja California differ from other populations, there is much overlap in linear measurements (the only varying characters so far identified) among birds from the rest of the range. Questions include whether all birds west of the Rocky Mountains are appropriately identified as brewsteri, differences among the remaining North American birds (sometimes referred to as candidissima), and the intraspecific status of Central and South American birds.
Range and status
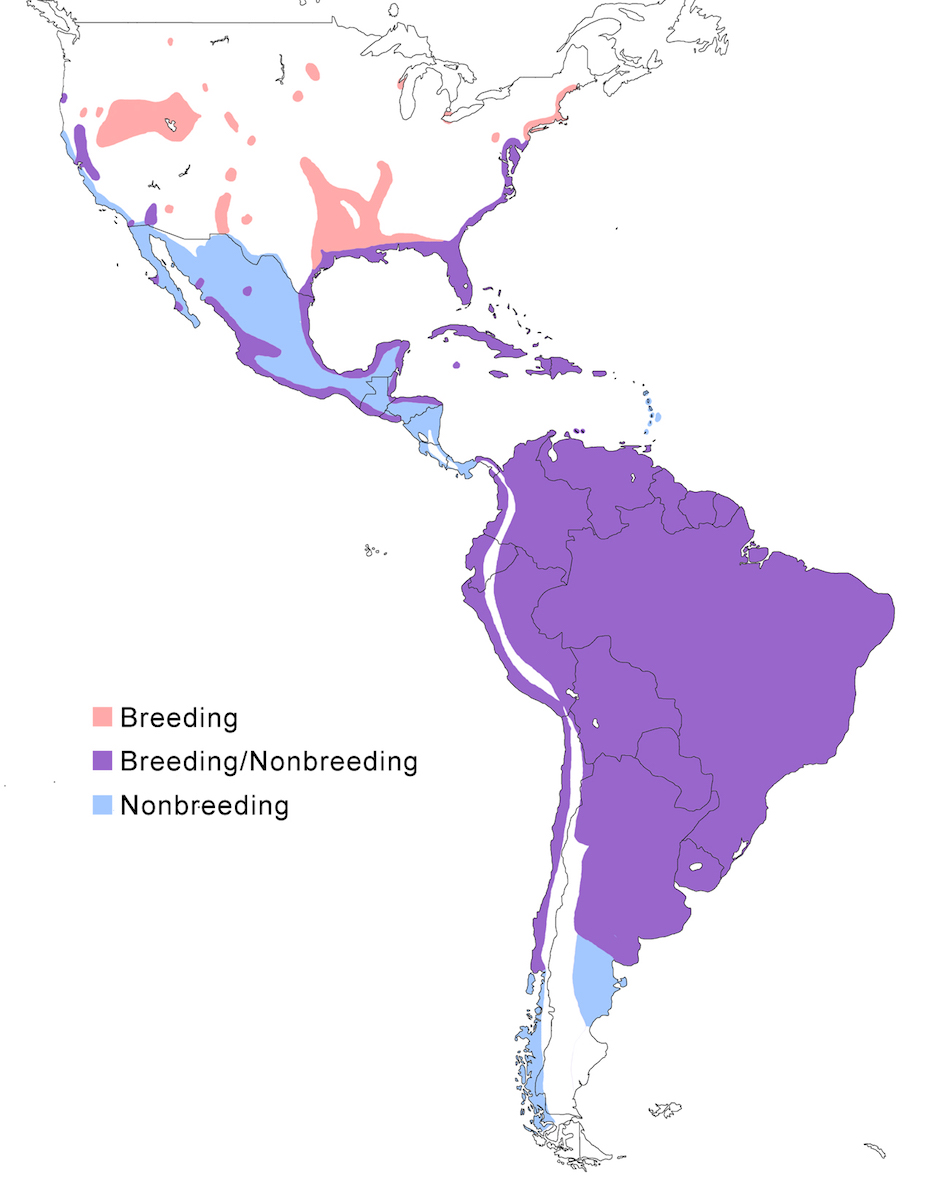
The Snowy Egret occurs in North America through Central and South America to Patagonia.
Breeding range: In North America, the principal breeding range is along the Atlantic and Gulf of Mexico coasts. Birds occur in summer in Nova Scotia but have not yet been seen to nest (Erskine 1992). They do nest further south along the coast from Penobscot Bay Maine (Drennan and Bowman 1993), New Jersey, Delaware and Chesapeake bays, to coastal and inland Florida and Texas. They nest inland in east and central United States in South Carolina (Belser and Post 1987), Pennsylvania, Ohio, south Ontario (Weir 1986, Curry and Bryant 1987), Wisconsin, South Dakota (Naugle et al. 1996), along the Mississippi and Arkansas Rivers, and in a block from Louisiana to east Texas to east Oklahoma to south Kansas. Breeds sporadically elsewhere. In the west United States, it nests in central Oregon, central California, south California at the Salton Sea and Colorado River, and in disjunct areas and pockets in several western states of Idaho, Montana, Wyoming, South Dakota, Nevada, Utah, Arizona, New Mexico. The breeding range continues down both coasts of Mexico where it breeds patchily in coastal wetlands (Massey and Palacios 1994, Becerril and Carmona 1997), and into coastal Central America, where it is currently documented as breeding in Costa Rica, Panama but is assumed to breed elsewhere. In the Caribbean islands it breeds in the Bahamas, Greater Antilles, Virgin Islands, Cayman Islands, Guadeloupe, Antigua, Barbados, Bonaire, Aruba, Curaçao, Trinidad and Tobago. In South America, it breeds in extensive and fairly inaccessible wetlands through Colombia, Venezuela, Suriname, Brazil (Do Nascimento 1999), as far south as Valdivia, Chile, on the Pacific coast, Cordoba, Argentina, in the interior, and Buenos Aires, Argentina, on the Atlantic coast. Its actual distribution in this vast area is not well documented. And it likely it patchily distributed in inland South America as it is in North America.
A single subspecies, thula, occupies most of this range. Brewsteri occurs in west North America; its northern limits are unclear.
Nonbreeding range: The usual nonbreeding range extends along the Atlantic and Gulf of Mexico coast from south New Jersey through all of peninsular Florida to Texas (coastal and inland along rivers). Birds are recorded wintering as far north as Maine. On the Pacific coast the non-breeding range includes Oregon, California, Arizona (lower Colorado River valley), throughout Mexico, to coastal Panama and Costa Rica. Birds winter in Bahamas, Greater Antilles, and Lesser Antilles to Trinidad. Birds occur year round in the rest of the breeding range in South America, but movement patterns are not understood. North American birds occur in Colombia, Venezuela and Guyana, but likely other areas as well. Important wintering areas for North American breeding birds include south east United States, Bahamas, Cuba, and parts of the Gulf and Pacific coasts to Central America (Mikuska et al. 1987). In south South America, birds winter south of breeding range to Patagonia, Argentina. They have been reported as far as the Strait of Magellan (Wheelwright 1978).
Migration: The species is partially migratory in North America, with most northern birds and some that nest further south migrating for the boreal winter. Snowy Egrets from north east and east of North America migrate beginning in September and October flying along the coast to Florida, the Gulf of Mexico coast, the east Caribbean, and north South America. One eastern bird was reported from Panama, but this might be unusual for the population. Part of the population breeding in the southeast United States is sedentary. Mid-continent North American Snowy Egrets migrate to the Gulf Coast and through Mexico to Central America. Egrets from western United States are partially migratory, some remaining in central California, others winter along the Pacific Coast to Baja California, central Mexico, to Central America (Ryder 1978, 1998, Parsons and Master 2000). Some North American birds, mostly juveniles, remain in their wintering areas into the next breeding season (Parsons and Master 2000).
Post-breeding dispersal and vagrancy lead to birds occurring outside their usual range. In North America, dispersal records occur across south Canada and elsewhere in the interior and, similarly in South America. It occurs on ocean islands including regularly in Bermuda (Amos 1991), Falkland Islands (Anon. 1989), Tristan da Cunha Islands, Azores (de Heer and Bos 1989, Alstrom and Colston 1991), Hawaii and American Samoa (Scott et al. 1983).
Status: As far as is known, in the 19th century the Snowy Egret was abundant in the south and southeast United States and common as far north as New York. During the first quarter of the 20th century, it is probable that plume hunting reduced Snowy Egret populations throughout the Americas although credible numerical estimates do not exist. It was certainly extirpated from the South Carolina and much reduced in Florida. It likely suffered similarly south of the United States.
Protection, from about 1910 in the United States but later in Central and South America, allowed recovery. In the United States the population increased and breeding range expanded beyond the species’ known historic limits, including Oregon, Arizona, Colorado, South Dakota, Wisconsin, Ohio, Pennsylvania, New Jersey, New York, and Maine (Parsons and Master 2000).
In the mid 1970’s the coastal nesting population in east United States was about 159,000 birds (Butler et al. 2000). Populations in the continental interior are relatively small (Findholt 1984, Findholt and Berner 1988). The Tabasco wetlands of Mexico supported another 60,000 birds (Scott and Carbonell 1986). Given that Central and South America remain unreported, based on these data the range wide the population would be about 250,000 birds.
In recent years local populations have fluctuated, although data are incomplete. Decreases were recorded in Maine, Massachusetts, Delaware, Virginia; stability, in Maryland, South Carolina, Texas; both increases and decreases in Florida; increases in the West Indies, and huge increases in Louisiana - (Fleury and Sherry 1985, Raffaele et al. 1998, Parsons and Masters 2000, Butler et al. 2000). There is not now evidence supporting an overall decrease in North America (Butler et al. 2000).
Habitat
The Snowy Egret is primarily a species of coastal wetlands and large river basins and their mouths. Much of its range is coastal and extends inland along river drainages including the California Central Valley, Mississippi River Valley, the Everglades, Orinoco basin, Amazon basin, and Parana basin. This is not universal, however as it occurs in the continental interior in both North and South America.
It has broad habitat preferences. It uses both fresh water and salt-water wetlands, especially salt marshes, mangrove swamps, lakes, reservoirs, rivers, coastal lagoons, and bays. Its microhabitat preferences are open pools within denser marshes and swamps, small tidal creeks, open shallows at the edges of rivers, lakes, and lagoons. It requires protects swamp forest or bushes nearby for roosting and nesting. It also can feed in damp to dry grassland or dry bush, pasture, crayfish farm ponds, catfish farm ponds, rice fields, ditches, and canals.
Foraging
The foraging behavior and ecology of this species has been well studied and much is known about its approach to feeding (Willard 1977, Kushlan 1976b, Custer and Osborn 1978a, Caldwell 1980, 1981, Rodgers 1983, Kushlan et al. 1985, Kent 1986a, b, 1991, Itokawitz 1984, Itzkowitz and Makie 1986, Powell 1987, Hom 1983, Frederick and Collopy 1989, Master 1991, 1992, Frederick et al. 1992, Master et al. 1993, Ramo and Busto 1993, King and Leblanc 1995, Strong et al. 1997, Miranda and Collazo 1997). The Snowy Egret is a diurnal forager in open aquatic habitats. It is an active and aggressive feeder employing a variety of techniques. It forages primarily by Walking Slowly, and also by Standing and by Walking Quickly. It specializes in using its yellow feet to startle prey, using Foot Stirring, Foot Raking, Foot Probing and Foot Paddling to capture small fish and crustaceans in shallow water. It very typically forages from the air, using Foot Dragging, Dipping, Hovering Stirring, and Hovering, and can catch prey in midair (Toland 1999). It uses Bill-vibrating to attract prey. It uses a sequence of behaviors to fit the circumstance. A typical fast moving sequence is Run – Hop - Open Wing - Foot Stir, similar to but less exaggerated than the Reddish Egret. A slow sequence is Walk Slowly - Peer Over - Foot Stir, similar to the Little Blue Heron. These sequences illustrate that the Snowy Egret’s hunting is intermediate between these two species.
It is a highly sociable feeder. It feeds, travels, and roosts in single species and mixed species flocks with other herons. Flock feeding is highly characteristic. They are attracted to other egrets feeding. Despite its social aggregativeness, it is also highly aggressive (Kent 1986b). It defends its individual area within an aggregation, or attempts to encroach on other birds’ areas, using Upright, Alert Posture, Forward display, Crest Raising, Bill Jabbing, and Aerial Fighting with Aah, Arg, and Raah calls. This bird’s combat can appear quite vicious. It attacks the individual area of neighboring birds by Supplanting Runs and Supplanting Flights. It Robs prey from each other and other species. It feeds communally with other species as well. On dry land it follows livestock to pick up insects disturbed by their grazing, much like the Cattle Egret. It roosts with other species when not feeding and at night. Such roosts can number in the hundreds. Individuals do hunt alone or in small groups (Itzkowitz 1984).
Central to its sociality must be the symbiotic and commensal advantages of flock feeding, communal food finding, predator protection and social stimulation. Snowy Egrets, like other wading birds, are more efficient and have a higher capture rate feeding in aggregations than feeding alone (Kushlan 1977, Willard 1977, Master et al. 1993, Erwin 1983), because the active feeding of many birds renders prey more vulnerable by causing movements, deoxygenating water, reducing hiding places and so forth. Aggregative feeding permits birds to remain productively feeding at a site longer (Master 1992), presumably because the flock prolonged availability of prey. Feeding, roosting, flying to and discovering feeding sites in groups allow birds to take advantage of ephemeral food supplies and exploit diverse and changeable habitat conditions. Within the feeding flock, competition is great, and individual aggressiveness is used to secure resources.
It has the broadest range of described behaviors of any North American heron (Kushlan 1978a). Different feeding behaviors have different efficiencies under specific conditions (Kent 1987) allowing birds to make optimization choices not only with respect to habitat to be used, but also microhabitat and behavior. Behaviors such as Foot Stirring, using its distinctively colored feet, and Bill Vibrating are particularly useful for targeting small fishes that are attracted to disturbances (Kushlan 1973, Willard 1977). Egrets use aerial behaviors to access fish in deep water or scraps of dead fish in deep water (Kushlan 1972, Rodgers 1974). Prey robbing is both a threat and alternative feeding style that can increase opportunities even if not efficient (Kushlan 1978b). Snowy Egrets very typically increase their effectiveness by feeding in deliberate association with other species such as grebes, mergansers, or cattle. Standing is often efficient, but Walking, Foot Stirring and disturb and chase behaviors are more often used, which while less efficient can be more productive (Kent 1986a, 1987). However, they do not necessarily choose feeding site to optimize efficiency or intake (Itzkowitz 1984), but rather appear to adjust behavior to exploit prey that are available. Snowy Egrets have to forage for longer during the day than do similar species (Kent 1986b). The species appears to attempt to maximize its food intake as opposed to minimizing its energy expenditure, as contrasted, for example, with large herons.
The daily cycle of feeding and behavior use reflects diurnal patterns of prey availability and both diurnal and seasonal patterns of energy demand. In nonbreeding, the usual cycle is for aggregations to form in the early morning (Hom 1982, Erwin 1985, Master 1992). This is when the daily energy demand is high. In some circumstances aquatic prey can be highly vulnerable then, in that in shallow wetlands, nocturnal deoxygenation can force them to the surface (Kushlan 1976b, 1978a). This is when simpler feeding repertoires tend to be used. The largest aggregations tend to form in the morning, and as the day goes on the birds tend to disperse to smaller concentrations and begin to use more complex repertoires. They revisit patches during the day, increasing their foraging effort (Erwin 1985, 1989). In tidal situations, feeding timing and flock formation usually depend on the timing of falling tides, which expose prey in shallowing water. Seasonally, flocks respond to changes in food supply, for example when spring tides replenish prey supply in salt marshes (Master 1992). During breeding, foraging intensity (such as strike rate) increases (Parsons and Master 2000) and feeding continues longer into the day when the schedule is constrained by nest site attending duties.
The interaction of morphology, foraging behavior, prey captureability and habitat choice is highly constraining to the Snowy Egret. It has to work harder and longer than similar egrets to meet its energy requirements. When conditions are appropriate they are successful in meeting energy requirements sufficiently.
Its diet tends to be relatively restricted to small fish and crustaceans (prawns, shrimp, crayfish). Topminnows (Poecillidae), killifish (Cyprinodontidae), silversides (Menidia), topsmelt (Atherinops) are among the fish taken in various locations. Other prey includes fiddler crabs, crayfish, snails, aquatic insects, polychaetes, flies, grasshoppers, frogs, lizards, and snakes, and also food from dumps and sewage treatment plants.
Thus the diet resulting from the disturb and pursue feeding tactic is specialized (Kushlan 1978a, Parsons and Master 2000), composed mostly of fish and crustaceans that concentrate or otherwise become available under specific conditions of hydrology, tides, and water oxygenation. There is an inverse relation between the diversity of available prey and feeding behavioral diversity (Kushlan et al. 1985), suggesting that complex feeding strategies are relied upon during competition for limited resources. Coastal habitats are probably favoured throughout the range because tidal fluctuation provide diurnally renewable patches of small fish made available by the falling water levels. Inland areas used, such as rivers and tropical swamps, have seasonal water level fluctuations that similarly make food available.
Breeding
Over such a broad range from northern to southern temperate zones, the breeding season is variable. In the temperate areas Snowy Egrets nest in the spring, often quite early, March to May in the United States. In the tropics it nests in relation to the rainy season or other conditions affecting prey availability. Some dates are March to May in Puerto Rico, May to August in Panama and Trinidad, November to January in Ecuador and Chile.
It nests in trees and bushes on islands and on “islands” of vegetation within wetlands. Mangroves are preferred nesting sites in coastal tropics. Dredge spoil islands in the coastal lagoons of east North America have provided nesting habitat in lieu of natural sites. The Snowy Egret is highly colonial and typically nests in mixed species colonies, some very large. It nests in the core of the colony. Its nests are close together often less than 1 m apart. Because courting males are attracted to a displaying group, nearby nests tend to be at the same stage. Snowy Egrets nest with a variety of other species, including other herons, ibises, spoonbills (Ajaia), boobies (Sula), cormorants (Phalacrocorax), darters (Anhinga), frigatebirds (Frigata), and gulls (Larus).
Nests are made of sticks with reed, rush, or soft bromeliad (Tillandsia) as lining. Nests are shallow flat, sometimes elliptical platforms, 40 cm wide, 27 cm deep. They are placed 3-10 m from the ground. The nest is built by the female from sticks brought be the male.
Males select display sites and advertise, with full display of the extravagant plumes. The advertising male principally uses the Stretch. Birds doing low intensity Stretches tend to attract other males to form a displaying group. In the Stretch, the bird points its bill up and then pumps its head up and down from a few to up to 10 times, giving the characteristic repeated Wah call. Males mark and defend display and nesting territory with Upright, Alert Posture, Crest Raising, Raah calls, Forward, Supplanting Flights (only of a few meters), Face to Face Fighting, and Aerial Fighting. As courtship continues, pairing occurs. Birds are active in their courtship. Males perform Aerial Stretch, Circle Flight, Tumbling Flight, and Jumping Over displays.
Eggs are pale blue green. Size appears to be variable: 42 x 30 mm (north east United States), 33 x 26 mm (Chile), 43 x 32 mm (Trinidad), 40 x 31 mm (Surinam). There is limited evidence of replacement broods, but they will replace eggs lost during laying period. Clutch varies from 2-6, generally 3-5 in northern colonies to 2-3 in the tropics.
Both sexes incubate. Incubation is 22-24 days, beginning with the first egg. Asynchronous hatching of young leads to differential mortality, usually through starvation of youngest chicks. Both parents feed the young, by regurgitation, initially by dropping food on the floor of the nest and later directly to the young, who grab the parent’s bill crossways.
Chicks are altricial on hatching. They are brooded by both parents continuously for 10 days. Mass gain reflects feeding conditions during a year. Body and wings are feathered in 2 -3 weeks. They are able to clamber from the nest in 20-25 days (McVaugh 1972). Other feathering took 4 weeks. They can fly in less than a month from hatching.
Nesting success is variable from place to place and season to season. The principal cause of nestling death and nesting failure is starvation due to a failure of the food supply, either in the short term or due to habitat alteration (Parsons and Masters 2000). Black Crowned Night-Herons, crows, snakes, and owls cause losses of eggs and chicks. Exposure deaths due to rain and storms also occur (Dindo and Marion 1992). For most colonies reported nesting success was 1.6 to 3 young per nest.
Population dynamics
First breeding is probably when approaching second birthday, but nesting by first year birds is suspected. Mortality of young after dispersal from natal colony is high, 35-60% (Erwin et al. 1996b). Mortality during first year is 71.6%. Annual adult mortality is 52.4%. Longevity record is 22 years.
Mortality risks of adults are predation, food failure, and contaminants. Predation risk is especially prevalent in the tropics (Caldwell 1986). Chlorinated hydrocarbon pesticides have been implicated in reproductive failure in Idaho probably obtained in Mexico (Findholt 1984), although range wide in the United States most studies have found below-effect contaminants levels (Mora 1996). Tissue and feather concentrations of mercury in Snowy Egrets in east United States are sufficient to raise concern (Burger and Gochfeld 1997).
Conservation
Uncertainty about recent population trends in the USA suggests the need for a range-wide survey, which should be readily possible given the conspicuousness of the birds. An action plan for this species within the southeast USA is desirable in that it has shown a tendency for population shifts. Provision of a suitable dispersion of nesting and feeding sites is crucial, especially given the tenuous future of crayfish aquaculture. In North America, tri-national conservation efforts need to be organized to monitor pesticide burden and determine any populational impact of contaminants. Important nesting and feeding sites for the species need to be identified and appropriately managed, both in North America and throughout its range. Coastal development is a threat to this species throughout its range, but especially in Mexico and Central America (Massey and Palacios 1994).
Research needs
A range-wide survey of the nesting population is needed over the east and south USA, designed sufficiently to detect changes since the mid 1970’s. Surveys should also be conducted through Central America and north South America to better assess population status and dispersion in those areas. The species’ distribution should be examined to detect important nesting, foraging, migration, and wintering areas. Colony site dynamics, colony shifts, philopatry, and relationships of food and habitat quality to nesting success require additional study. This is especially the case in declining colonies in the northeast USA, which need to be examined to determine causal factors. The foraging behaviour of the species presents additional opportunities to address fundamental questions of feeding ecology, both inside and outside of the nesting season. Given its simultaneous gregariousness and aggressiveness, it is a species that may show interesting patterns of individual choice of behaviours, if individuals could be identified and followed. Patterns of intra-specific geographic variation over its extensive North to South America range deserve closer scrutiny to determine the genetic populational structure, which could be of importance in designing conservation strategies.
Overview
This is a species characterized by its sociality yet also by its aggressiveness. Its huge repertoire of social and feeding behaviours can be mixed and matched to accommodate the situation at hand. The small prey sought are usually mobile species that require high-energy foraging to obtain. But this egret is small, light, nimble, and quick and it appears to maximize its prey intake, rather than conserve its own energy. Individuals take advantage of ephemerally available food supplies. Populations undertake local movements and the species’ range has changed with time, such as its shift from the east USA to Louisiana to take advantage of the development of seasonally drying crayfish farms. This is an adaptable species that exhibits diverse behavioral, social and population approaches to increase energy intake.