Striated Heron
Butorides striata (Linnaeus)
Ardea striata Linnaeus, 1758, Syst. Nat. 10th Ed., p. 144: Surinam.
Subspecies: Butorides striata sundevalli (Reichenow) 1877: Galapagos Archipelago; Butorides striata atricapilla (Afzelius)1804: Sierra Leone; Butorides striata rutenbergi (Hartlaud)1880: Mohambo, northern Madagascar; Butorides striata brevipes (Ehrenberg) 1833: banks of Nile and coasts of the Red Sea; Butorides striata crawfordi (Nicoll) 1906: Assumption Island; Butorides striata rhizophorae Salomonsen 1934: Mayotte, Comoro Islands; Butorides striata degens Hartert, 1920: Praslin Island, Seychelles; Butorides striata albolimbata Reichenow, 1900: Diego Garcia, Chagos Archipelago; Butorides striata chloriceps (Bonaparte) 1855: India, restricted to Hitaura, Chisapani, Garhi district, Nepal; Butorides striata javanica (Horsfield) 1821: Java; Butorides striata amurensis (Schrenck) 1860: Amurland, Amur Valley, eastern Manchuria; Butorides striata actophila (Oberholser) 1912: North Pagai, western Sumatra Islands; Butorides striata spodiogaster Sharpe, 1894: Andaman and Nicobar Islands; Butorides striata carcinophila Oberholser 1924: Casiguran, Luzon, Philippines; Butorides striata steini Mayr, 1943: Dilly-Dìli, Timor; Butorides striata moluccara Hartert, 1920, Buru, Moluccas; Butorides striata idenburgi Rand, 1941: Idenburg River, Dutch New Guinea; Butorides striata stagnatalis (Gould) 1848: Port Essington, Northern Territory; Butorides striata flyensis Salomonsen, 1966, Lake Daviumbu, southern Papua New Guinea; Butorides striata macrorhyncha (Gould) 1848: east coast of Australia = Gosford, New South Wales; Butorides striata solomonensis Mayr, 1940: Vangunu Island, Soloman Islands; Butorides striata patruelis (Peale) 1848: Tahiti.
Other names: Little Heron, Green-backed Heron, Little Green Heron, Red Heron, Mangrove Heron, Red Mangrove Heron, Red Mangrove Bittern, Johnny Mangrove, Mangrove Jack, Thick-billed Heron, Galapagos Heron, Lava Heron in English; Garcita Verde in Spanish; Socozinho in Portuguese; Heron vert in French; Streifenreiher in German; Mangrovereiger in Dutch; Зеленая кваква in Russian; Kokokan laut in Indonesian; Bakaw in Pilipino, Bakaw Itim in Tagalog (Philippines); Sasa-goi in Japanese; Lü lu in Chinese.
Description
The Striated Heron is a small, stocky dark backed heron with a thick neck, relatively heavy dark bill, thick short legs, and grey neck.
Adult: The adult typical Striated Heron has a glossy green black crown with a short erectile crest. The irises are orange yellow. Lores are dull yellow green above and black on the lower portion. The bill is dark, the upper being brown black and the lower dusky green, lighter at the base. The dark line extends from the bill to under the eye. The sides of the head, side of neck, and breast are light grey to buff, with considerable geographic variation. The back is dark grey with a green cast, with elongated feathers especially toward the tail. Upper wings are grey black with obvious buff edges to feathers. Flight feathers are black, with edges buff colored. The chin and throat are white, with a row of central grey spots. The throat and the center of the upper breast are streaked vertically in a rufous, rust or chestnut band streaked with white and edged in grey. Underparts are brown grey to grey in most forms. The legs are olive grey in front and yellow green behind.
In breeding, the head, erectile crest and back become glossy blue black. The irises turn deep orange in courtship. The bill becomes glossy black, but remains less so in females. The lores turn blue black or cobalt blue. The legs turn glossy orange, even in the grey-legged sundevalli (Kushlan 1983). However bills of red or pink have also been reported.
Variation: Females are slightly smaller and duller than males. Variation in this species is widespread, complex, and far from understood. In the Old World, polymorphic variation occurs among grey, rufous and intermediate forms. Rufous forms have head (other than crown), neck and back rufous brown, with feathers fringed slightly darker. The undersides are light rufous brown. Intermediate forms have more white on the foreneck, light grey underparts, and greyer upperparts. In the New World, variation includes birds with brown grey necks widely reported throughout South America. There is also variation in the undersides, with some birds being extremely white. While a matter of some controversy, birds intermediate between striata-type and virescens-type occur where their ranges meet in southern Central America, south Caribbean islands, and the north coastal South America (Payne 1974, Hayes in prep.).
Described geographic plumage variation is the largest of any heron, recognized by 23 currently accepted subspecies based mostly on size and neck and head color. Many of these variations are found in island populations, but subspecies are also described from within the Asian and Australian mainlands. Some populations are well differentiated whereas other intergrade into each other (Payne 1974, 1979, Schodde et al. 1980).
Butorides striata striata has a dark grey neck and green grey back plumes. However, birds with brown grey necks occur throughout South America (Payne 1974). South American birds also differ in the amount white on the undersides varying from white, to intermediate, to grey, the whitish birds once being recognized as the subspecies cyanurus. Sundevalli is dimorphic with herons that are entirely dark and also grey birds similar to those from South America, with perhaps neck streaks being more grey brown than rufous. The dark birds are dark grey with back plumes of blue green and grey legs. Intermediate birds also occur.
The African atricapilla has a dark grey neck and differs from South American Striated Herons in lacking the brown grey morph, in having more distinct rufous on the upper breast outside of the lower neck streaks and also rufous rather than grey brown streaks on the lower neck (Payne 1974). Brevipes is darker than atricapilla, with more bronze and less bright green back and crown. Solomonensis is brownish. Patruelis has light buff neck and undersides. Amurensis has a light grey neck. The closely related rutenbergi from Madagascar is slightly darker. Chloriceps, javanica, and carcinophila are similar to each other. Javanica is less grey and bluer than chloriceps. Carcinophila has very dark neck and breast. Stagnatilis is a pale dimorphic form, with rufous and grey types, and relatively long bill. Macrorhyncha is a dark form with rufous underparts, and a relatively short bill. Flyensis is a pale bird with green gloss to back plumes and relatively short bill. Idenburgi is a small pale bird, very light on its undersides. Molluccarum is small and dark, with relatively short bill.
Juvenile: The juvenile is an overall brown bird with white spots. It has a brown black crown with narrow buff to white streaks and a slight crest. The upper bill is brown black above, the lower bill, yellow. Irises are yellow and lores are yellow green. Sides of the head and upper neck are brown heavily streaked with buff white. The chin and upper foreneck are white, showing brown tips to the feathers, turning to dark brown with buff streaks on the lower foreneck. The back and wings are olive brown spotted in buff white. The underwing is light grey. Flight feathers are dark grey with a green cast, tipped in white. The underparts are brown to dark brown streaked or spotted in buff. Legs are yellow green, greyer on the front. Juveniles gradually lose the streaking on their head, neck, and underparts and the white on the flight feathers.
Chick: The hatchlings are covered with grey down on upper sides. Down is longer and darker on head forming a crest. Irises are green yellow, becoming yellower or white. Skin on head is green; the lores are yellow green. Eye ring is green yellow. Bill is yellow with dark tip. Throat is white. Down is light grey on undersides. Legs are green becoming yellow green in fledging.
Voice: The voice is not well characterized, especially compared to that of the Green Heron. It is reported to be relatively silent bird and so appears to be far less vocal than the Green Heron. The primary vocalization is the “Keeuuk” call, used as an alarm, flight and advertising call. It is also rendered as “k-yek”, “baaek”, “tyong”, “tyah”, “tkyah”, “skuk”, or “tchack”. Skow calls are given by displaying males (at least in the Galapagos – Kushlan 1983). A sneezing “Tech-aah” call and a “Hoo” call are reported in courtship. In sundevalli, the female answered the male’s stretch display with a soft Coo call. “Tchee-unk” is used on arrival at the nest. Other calls, especially squawks and croaks, have been reported but their context is not documented.
Weights and measurements: Length 35-45 cm in South America, 40-41 cm in Africa, and 43 cm in Australia. Weight: 193-235 g (Africa).
Field characters
The Striated Heron is identified by its thick grey to rufous neck, black cap, relatively large bill, and dark grey back. It flies with slow wing beats and legs extended beyond the body making it appear short tailed. It is distinguished from the Green Heron by its grey to rust not chestnut neck. It is distinguished from other herons by its combination of dark crown, relatively large bill, uniform color pattern (although geographically variable) of the sides of head and neck, yellow lores, yellow to orange legs, crouched feeding posture, and behavior including tail flicking. It is distinguished from the dark little bitterns (Dwarf and Black) by being larger and lighter colored (and lacking the white or yellow throat stripes) and from other little bitterns by its larger size, dark cap and grey back. The rufous form is distinguished from the adult Rufous Night-Heron by being smaller, having dark (not white) underparts, and green (not yellow) legs.
The immature Striated Heron is identified by its dark cap and dark spotted back. It is probably not distinguishable from the Green Heron, although it may tend to be greyer on the neck (F. Hayes pers. comm.). The Striated Heron is distinguished from immature night herons and Rufous Bellied Heron by being darker with less spotting, with a darker cap, and being much smaller with longer neck and slighter bill. It is distinguished from immature pond herons by being darker, lacking head and breast streaking and having dark wings. It is distinguished from the juvenile small bitterns by its larger size, dark crown, and darker upper parts.
Systematics
As noted in the Green Heron account, the systematic position of the Butorides herons has not been clear. Butorides appears to be closely related to Ardeola, and together these species are most closely related to the Ardea herons.
Species level systematics of the Butorides has been a matter of considerable study. In this book striata is recognized as distinctive from the North American virescens based principally upon several studies, the conclusions of the North American Check List Committee (AOU 1998) and the trend of molecular evidence suggesting distinctiveness of the order generally attributable to species differences (K. McCracken and F. Sheldon pers. comm.). But these findings are not yet conclusive and cannot be until the breeding birds are thoroughly studied in the wild in their zones of overlap. Fortunately, new information is emerging (G. Alvarado pers. comm., F. Hayes pers. comm.). Although controversial, further examination of available specimens suggests that an extensive hybrid zone in the south Caribbean islands, north South America and south Central America. The variability and intermediacy in the zone of contact suggests that they interbred freely (Hayes in prep.). Although not definitive, requiring additional molecular, behavioral, and perhaps morphological study, these finding suggest that by traditional concepts, the two Butorides would be considered conspecific. So it is likely that the question of specific distinctiveness of the two forms is not yet settled. Additional questions remain also as to the relationship between the South American and Old World forms and similarly, within the Old World, as to the relationships among the mainland and many island forms.
Individual variation also occurs. In the New World, striata occur in various places with neck coloration ranging from grey to brown; such variability is not shown in African or Asian birds (Payne 1974). Grey and rufous morphs appear among Old World populations, and additional individual variation occurs as well. There appears to be clinal variation among the Old World populations that may not be caught adequately by the present array of recognized subspecies.
At the subspecific level, the considerable range of variation in color and morphometrics are recognized in 23 subspecies, many of which are island populations. In the New World, two subspecies are recognized, the nominate form and sundevalli, a particularly distinctive, dimorphic island population with large feet, sturdy legs, and a majority of birds with an all-dark grey plumage, unique in the group. Sundevalli has often been considered a separate species. However, South American color forms are common on the Galapagos, and it is likely that there is interchange of birds between South America and the Galapagos. Despite the morphological differences, there has not been found yet to be molecular differentiation between the Galapagos birds and mainland birds (K. McCracken pers. comm.).
In the Old World, 21 named forms are recognized. In this book, we differ from the Old World subspecies listed in Hancock and Kushlan (1984) in following the findings of Schodde et al. (1980) who recognized five Australasian subspecies: stagnatalis including rogersi and cinerea (of Hancock and Kushlan 1984), macrohynchus including littleri (of Hancock and Kushlan 1984), flyensis (not recognized in Hancock and Kushlan 1984), idenburgi, and moluccara including papuensis (of Hancock and Kushlan 1984).
As noted above, variation over the Old World range of the species deserves additional attention. Much of the plumage variation seen is individual and developmental, and may not have a geographic component. Certainly geographic variation is also substantial given the array of subspecies currently recognized. Despite the material already available, study of geographic variation is hampered by the lack of specimens for places and seasons. Molecular evidence may hold the best clues for understanding the relationships among forms. It may well be that some currently recognized subspecies may better be considered full species. The systematics and evolutionary relationships among Butorides herons are clearly one of the more interesting research issues within the Ardeidae.
Range and status
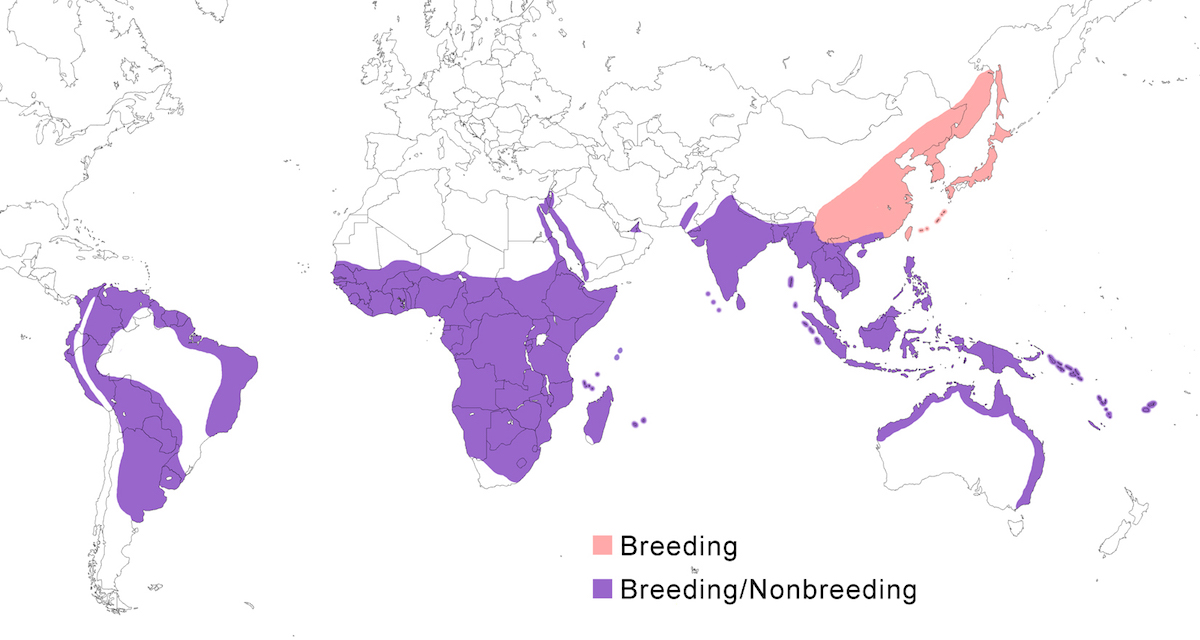
RANGE: The Striated Heron is one of the more cosmopolitan herons, occurring in South America, Africa and Madagascar, Indian Ocean islands, south and east Asia, the East Indies, Australia and Pacific islands. Information on range relative to subspecies is from Payne (1974, 1979) and Schodde et al. (1980).
Breeding range: Striata is the South American form. It breeds in east Panama, Colombia, Venezuela, Trinidad, Guyanas, Ecuador, Peru, Bolivia, Brazil, Paraguay, Uruguay, Argentina (to Buenos Aires, La Pampa). Sundevalli breeds in the Galapagos Islands.
Atricapilla breeds in Mauritania, Senegal, Gambia, Guinea-Bissau, Guinea, Sierra Leone, Liberia, Ivory Coast, Mali, Burkina Faso, Ghana, Niger, Nigeria, Chad, Cameroon, Gabon, Principe and São Tomé; also in Sudan, Ethiopia, Somalia, Kenya, Tanzania, Uganda, Rwanda, DR Congo, Angola, Botswana, South Africa, Zimbabwe, Zambia, Malawi, and Mozambique (van Nierkerk 1992, Turner pers. comm.). It is probably this subspecies that is reported to breed in Aswan, Egypt, as opposed to the Red Sea form, brevipes (Dijkestra 1997).
Brevipes breeds on the Red Sea coasts of Egypt, Sudan, Eritrea, Djibouti, Somalia, Yeman and Saudi Arabia (Baccetti 1983, Stagg 1984, Goodman and Storer 1987, Hoath et al. 1997, Porter and Saghier 1998).
In the Indian Ocean, rutenbergi breeds on Madagascar; crawfordi breeds on Aldabra, Assumption, the Amirantes, Cosmoledo, Astove and Farquhar; rhizophorae breeds on the Comoro Islands; degens breeds on the granitic islands of the Seychelles; and albolimbata breeds on Diego Garcia, the Chagos archipelago and the Maldives.
Chloriceps breeds in India (Sind, Punjab, Kasmir, east to Assam, Manipur, Laccadive Islands), Sri Lanka. Javanica breeds in Myanmar, Thailand, Malaysia (peninsular), Indonesia (Sumatra, Java, Kalimatan, Bali). Birds from Mauritius and Rodgrigues islands (Rowlands 1987) are assigned to this subspecies but are difficult to distinguish from chloriceps. Amurensis breeds in Russia (Amurland, Ussuriland, Sakhalin Island), North Korea, South Korea, northern China (Heilongjiang to Shandong), Japan (Honshu south, Ryukyu and Bonin islands).
Actophila breeds in south China (Sichuan, Yunnan, to south Shaanxi, Hainan), north Vietnam, north Laos, north Thailand, north Cambodia, north Myanmar.
Carcinophila breeds in Taiwan, Philippines (Luzon, Negros, Cebu, Samar, Mindanao), Indonesia (Sulawesi and nearby islands). The birds from Taiwan and Sulawesi are assigned to this subspecies on the basis of their darkness. Steini breeds in Indonesia Lesser Sunda Islands (Sumba, Flores, Alor, Timor). Moluccara breeds in west New Guinea and nearby islands including the Mollucas (Schodde et al. 1980). Idenburgi breeds in Irian Jaya (Schodde et al. 1980). It has not been confirmed if this is the subspecies of birds from north Papua New Guinea. Spodiogaster breeds west Sumatran islands (Indonesia), Andaman and Nicobar Islands (India).
Stagnatalis breeds in coastal mid and north west Australia and north Australia (Schodde et al. 1980). Macrorhyncha breeds in coastal east Australia (Schodde et al. 1980). Flyensis breeds on the south coast of New Guinea (Schodde et al. 1980). Solomonensis breeds in New Ireland, Solomon Islands (Schodde et al. 1980), Vanuatu, west Fiji Islands. Patruelis breeds on Tahiti, Society Islands.
Nonbreeding range: Most subspecies are sedentary. Northern populations are migratory or partially migratory in nonbreeding season. Amurensis occurs in nonbreeding season in south China, north Indochina, Taiwan, Philippines, Malaysia (north Borneo), Palau Islands. Actophila is partially migratory. Some remain in the breeding range (Desholm and Wegelberg 1997) but also occur in nonbreeding season in as far south as Nicobar Islands (India), and Indonesia (west Sumatra islands, west Kalimatan). Some atricapilla populations show local rain-dependent movements (e.g, Gambia - Barlow and Wacher 1997), while in South Africa erratic seasonal movements have been noted during flood years (Brown et al. 1982, Becker 1984, de Swardt 1992, Esterhuizen 1994).
Migration: Migration patterns for amurensis and actophila are poorly known. Similarly local movement patterns are poorly documented, other than the coming and going of birds from an area. Although dispersal is not as dramatic as for some other herons, it has colonized many islands. Islands dispersal records include Christmas Island (Indian Ocean) and Tonga (Gill 1990).
Status: Overall this is a widespread and common species where it occurs. It is common and widespread in Africa, although populations are not estimated (Turner 2000). It is abundant in some west African mangrove swamps, often nesting in the hundreds (Turner pers. comm.). A population estimate for Tanzania is 10,000-15,000 birds (Baker and Baker in prep.). It occurs throughout Madagascar, but is rare on the High Plateau. It is widespread on the Comoros and is relatively common to abundant on the Seychelles, where mangroves remain intact (J. Gerlack pers. comm.). While present on the Mascarene Islands it is not extirpated from Reunion.
It is widespread and abundant in east Asia (Lansdown et al. 2000). It has a limited range in Australia, in mangroves. It status is unclear there, but local reductions have occurred (Maddock 2000). It occurs sparsely in coastal areas of New Guinea. It has declined in Tahiti over the past century, now numbering about 100-120 individuals (Monnet and Varney 1998).
It is widespread in South America, although status is little known (Morales 2000). The subspecies on the Galapagos is common.
Habitat
The Striated Heron uses a variety of inland and coastal habitats, and ocean islands. It especially uses edges—small and large brushy river edges, river swamps, the edges of forested rivers and streams, lake margins, salt flats, woods, sand, muddy or rocky shores, pools, and in human made habitat such as rice fields, canals, and ponds. It forages typically at the edge pools, streams and channels, often from perches overhanging the water. Over much of its eastern range, it is a mangrove heron seldom found far from the coast and particularly using mangrove swamps. In Australia it is so restricted to mangroves that inland occurrences are notable (Gynther 1995, Britton and Britton 1996, Mitchell 1996). It occurs from sea level to considerable altitude, up to 4,000 m in the Andes (Fjeldsa and Krabbe 1990).
Foraging
The Striated Heron is a skulking predator feeding primarily in and under bushes adjacent to pools and watercourses venturing also onto adjacent mudflats and similar open areas. It characteristically feeds solitarily, defending its feeding site and sometimes a larger territory. In southern Africa, they occur along a river at a density of only 1.9 birds per 13 km transect (Allan and Davies 1999). It uses its crest to communicate its aggressive and territorial intentions, especially in response to other herons. On occasion, it will feed in loose groups and among aggregations if prey is especially available. It feeds by day, night or in the evening, and is attracted to lights to feed.
It feeds primarily by Standing in shallow water or next to the water. It feeds in a characteristic Crouched posture, with its body parallel to the water. The head is withdrawn or extended as it waits for long period between strikes, measured at 29 and 42 minutes per successful strike (Oliver 1995). It also Stands on branches overhanging the water and stabs into the water, sometimes submerging itself. It Walks slowly in very shallow water, along the shore, or along branches. It is so slow that its foot may be kept raised for 30 s between steps. Its rate of progress has been measured at 1 m/ min (Recher et al. 1983). In one study the number and frequency of strikes during Walking far exceeded that in Standing (Oliver 1995). It uses Standing Flycatching. It feeds by Walking Quickly and Running in such situations where active feeding is more successful than standing (Oliver 1995). It also uses Feet First Diving and Plunging (Davis 1983). Characteristically it flicks its tail up and down, at almost any time. It also assumes a Bittern posture to hide itself.
Reports of Striated Herons using Baiting are frequent and widespread (Walsh et al. 1985, Foxall and Drury 1987, English 1987, Crous 1990, Kurosawa and Higuchi 1993, Higuchi 1986, 1988b, Richards 1992, Robinson 1994, Pyle 2000, Hayes and Rooks 2001). Japanese herons particularly are noted to have used a wide variety of baits (Higuchi 1988).
The Striated Heron is primarily a fish eater (e.g., Niethammer et al. 1983), but it is also opportunistic. Fish include a wide array of species Belone, Periophthalmus, Boleophthalmus, Puntius, Mystus, Gobius, Mugil, Anbassis, Gambusia. They also eat insects (water beetles, dragonflies, grasshoppers), earthworms, polychaetes (Nereis), crustaceans (Metapenneus, Scylla, Portunus, Varuna, Macrobrachium), frogs (Rana), reptiles, and birds (Quelea) (Stocker 1994). Striated Herons also may eat vegetation (Beltzer 1983).
Breeding
The nesting season varies geographically, but tends to be in the rainy season. It is March–June in Java, November–April in north Australia, September–January in west Australia, and September–December in south Australia. In Africa the breeding season is diffuse, with nesting recorded in almost every month of the year but with well-defined peaks during the rains (Turner pers. comm.). In West Africa, while there are some dry-season breeding records, egg laying peaks during the July–September rains. In East and southern Africa, breeding has been recorded year round, with peaks in April–June, September–October, and again in December–January following periods of prolonged rainfall. On islands in the western Indian Ocean, breeding is generally September–January. In Tahiti 22 clutches were laid in nine different months (Monnet and Varney 1998). The situation in the Galapagos is typical of the coastal tropics (Kushlan 1983). Nesting begins with the onset of the rainy season, but herons may nest three times annually and so the nesting timing of individuals can be variable throughout the season (Kushlan 1993).
They nest in shrubs, bushes and trees usually overhanging water, in well-concealed locations. However they occasionally nest over dry land. Plants used especially include mangroves (Rhizophora, Avicennia) and also Allocasuarina, Myoporum, Callistemon, Hibiscus, Casuarina, Syzygium, Inga. Unusual sites are also used, such as a TV antenna in Japan (Kobayashi and Hironaka 1992).
Most Striated Herons nest solitarily, most typically spaced along the shoreline of a river, linear swamp or seacoast, where they nest within their defended territories (e.g., Kushlan 1983). However, they also nest in various degrees of proximity from loosely spaced nests and single species groups of 5-15 nests to breeding aggregations in the hundreds, such as have been reported from West Africa. They tend not to nest with other species, but when they do they tend to nest away from the main aggregation.
Nests are small, shallow, loosely constructed, often flimsy, structures, about 20 to 40 cm in diameter. Nests are typically low to the water, about 1 m above the surface. They are made of twigs and, as far as is reported, unlined. Building takes up to 14 days, but the parents also continue to improve the nest during nesting.
Courtship behavior has been little documented. Courtship begins when the male chooses an advertising site, often an old nest. Territories are defended by the bird’s assuming an aggressive presence at the nest site, emphasized by crest raising as well as Pursuit Flights. Early displays include Circle Flights and Pursuit Flights. Males give the Skow call from the display site. Pairing birds give simultaneous Snap and Stretch displays, which lack a side-to-side swaying component. The female gives a Coo call as the male does the Stretch display. Observations of the Stretch display in sundevalli (Kushlan 1983) differ from those of the Green Heron by lacking the swaying motion and by the Stretch being performed mutually by both males and females prior to pairing. Mock Preening and Allopreening are common between members of the pair. The Crest Raising occurs upon alighting and is undoubtedly an important part of the landing display.
Eggs are pale green blue, becoming duller as incubation proceeds. They average 41 x 31mm in Australia, 37 x 28 mm in Africa, 37.7 x 29 mm in Madagascar. The normal clutch is 3-5, range 1-7. Average clutch size is 2.37 in east Africa, 2.7 in Zimbabwe, 2.6-2.8 in South Africa, 3 in Madagascar and only 1.05 in Tahiti (Monnet and Varney 1998). Eggs are laid at two-day intervals.
Incubation is by both sexes, beginning with the second egg, although there is evidence for beginning with the first egg. Incubation is recorded as 21-22 in Galapagos, 21 days in South America (Mosso and Beltzer 1992), 21-25 in Africa and Australia, 25-29 in Tahiti.
Hatching is asynchronous. Young hatch over period of 3-4 days. Chicks at hatching are semialtricial, having limited movement. Chicks grow quickly and climb about on branches by one week. Young grasp parent’s bill and are fed by regurgitation. Both parents attend the young, which leave the nest in 14 days (Mosso and Beltzer 1992). Adult care appears to continue in a family group for some time after the young have left the nest.
Disappearance of eggs, usually attributed to predation, may be high in some areas. Nest predators include water dragons (Physignathus), shrikes (Colluricincla), crows (Corvus), and eagles (Haliaeetus). Nests are also lost due to high tides and storms. Survival of young in East Africa is reported at less than 2 per nest (Brown et al. 1982).
Population dynamics
The usual age at first breeding is probably at two years, but birds in immature plumage appear at nesting colonies and so may be breeding or attempting to breed in the first year. Morality is poorly documented, with only a few scattered reports of predation on adults. The demography and population biology is little understood.
Conservation
Loss of mangroves in Australia and Seychelles and elsewhere in its range is creating cause for concern. This is a polymorphic species with many island forms. Each of these forms has its own set of conservation issues that need to be addressed. Conservation of each genetic population is crucial.
Research needs
There is surprising little known about the biology, especially the behaviour of this species. Its courtship behaviour, vocalizations, and aggressive behaviour need especially to be documented, given the taxonomic uncertainty in the group and the substantial knowledge base existing on the behaviour of the Green Heron. Basic comparative information is needed on populations in South America, Africa, Asia, and Australia. Foraging, habitat selection, and prey taken all need to be better documented throughout its extensive range. Documentation of population sizes and trends also are needed throughout the range. The present status of island populations especially needs to be documented. Perhaps the most intriguing and important research need is for further systematic study of the various geographic populations. Determining the evolutionary relationships among the populations will require much additional molecular and behavioural study range-wide. Determining the genetic structure of the species is not only of biological interest but need to underlie a conservation strategy that protects distinctive populations and preserves the inherent variability in this widespread group of birds.
Overview
The Striated Heron is a solitary species, of mangroves, streams, ponds and lakes, and inland wetlands. It particularly uses the edges of watercourses, either wading in very shallow water or feeding from overhanging branches. It feeds quietly, with great patience, and skulks from place to place, often returning to favoured sites. It nests solitarily, or in very loose groups. Most populations appear sedentary, although some shift as the wet and dry seasons make habitats alternatively available and unavailable. This is a highly successful species, especially on islands and in mangroves swamps throughout the Old World tropics.