Whistling Heron
Syrigma sibilatrix (Temminck)
Syrigma Ridgway, 1878. Bull. U.S. Geol. and Geogr. Surv. Terr., 4, pp. 224, 247.
Ardea sibilatrix Temminck, 1824, Planches Color., Livr. 46, p. 271.
Subspecies: Syrigma sibilatrix sibilatrix (Temminck) Brazil and Paraguay; Syrigma sibilatrix fostersmithi Friedmann, 1949, Smithsonian Misc. Coll., 111, No.9, p. 1. Caicara, Monagas, Venezuela.
Other names: Garza Chiflora, Chiflón, Garciolo Real, Pacopaco, Garza Silbadora in Spanish; Mariafaceira in Portuguese; Héron flûte-du-soleil in French; Pfeifreiher in German.
Description
The Whistling Heron is a medium-sized grey heron with a strikingly colored yellow gold chest and neck.
Adult: The crown is dark to slate black featuring several distinctive, elongated black plumes, tipped with white or yellow. These rather rigid, lanceolate plumes emerging from the back of the head can be up to four cm long. Unlike most heron crests, these actually insert relatively far down the back of the head such that the crest encompasses the head and upper back of neck (see Hancock 1999). Lores and base of the bill are light blue to violaceous and the long slender bill is pink to red proximally and black distally to a third of the way from the tip. The iris is pale yellow. A distinctive feature is its yellow to buff neck and chest, which varies in intensity. The yellow in the plumage of this species is adventitious, being applied after feather growth, probably from the powder down and/or preen gland. In museum specimens the yellow typically fades toward white. Throat, upper neck, breast are white. Since these feathers remain white, it is likely that the yellow in other plumage requires a combination of adventitious color and feather structure. The predominant coloration of this species derives from the bluish-grey of its back, scapulars, and flight feathers. The median and lesser wing-coverts appear yellow and cinnamon, streaked black. Abdomen, flanks, tail and tail coverts are white. The legs (with scutellate tarsi) are greenish-black. Bill and lores brighten as breeding approaches; the bill becomes markedly red.
Variation: The sexes are alike as far as is known. The southern race sibilatrix has a very black crown, pinkish-cinnamon wing-coverts, and a light buff-olive neck and tends to be larger whereas the northern race fostersmithi is smaller in stature, and has a longer bill, a more slate crown, and honey-colored instead of cinnamon wing coverts with less streaking. A pale grey area is reported between a pinkish lower mandible and a cobalt to azure-blue upper mandible, although a reddish coloration is also reported (Cherrie 1916).
Juvenile: The plumage of the adults resembles that of the adult. The immature plumage is duller, neck feathers streaked, crown more slate colored, with narrower streaks on lesser wing coverts, and light grey neck and chest. Throat, sides, abdomen, and tail are white as in adult. The yellow in the plumage is acquired after the feathers are grown fully.
Chick: The chick has not yet been described.
Voice: The voice, after which this heron is named, is a distinctive, characteristic, far-carrying, melodious whistle, the “Kee” call. It is given in flight with the neck stretched and then partly retracted. It can be rendered as “kee, kee, kee”. Calls are well spaced. Also a slow drawn-out whistle may be given as the bird takes flight. There also is a peculiar high pitched and rapidly repeated call (Friedmann and Smith 1950), possibly the “hard metallic notes” described by Kerr (1950). Young communicate with adults with hisses.
Weights and measurements: Length: 53-64 cm. Weight: 521-546 g.
FIELD CHARACTERS
The Whistling Heron is identified as a grey heron with golden chest and black cap, which together distinguish it from other medium-sized herons. It is often seen alone or in pairs, in more open and relatively drier situations than most herons. It flies distinctively. It has rapid, duck like wing beats, gliding to its landing. Sick (1993) offers that its wings appear attached below the body axis. It seldom retracts its neck completely in flight, in the typical heron fashion.
Systematics
The affinities of the Whistling Heron have been long debated as it has been difficult to place within heron phylogeny. However, it is a typical heron, related to the Egretta (Sheldon 1987, McCracken pers. comm.). It likely is most closely related to the Capped Heron (McCracken pers. comm.). Earlier debate centered on whether it was better regarded as a day or night heron, but the affinity of Syrigma to the egrets is well supported by behavioral, morphological, and biochemical evidence (Humphrey and Parkes 1963, Short 1969, Kahl 1971, Payne and Risley 1976, Kushlan et al. 1982, Sheldon 1987). The only question is how closely it is related to Egretta. Interestingly, molecular data suggests it is closer than does evaluation of osteological data (Sheldon and Whittingham 1997), probably due to its secondary adaptation for semi terrestrial foraging.
Because of its apparent distinctiveness and as a result of its specializations, the species has historically been placed in its own genus. Neither recent morphological nor biochemical evaluation provides evidence to dispute such a distinction. Its combination of rigid, lanceolate head plumes, lack of scapular plumes, scutellate tarsi, possession of both day and night heron skeletal features, and measurement proportions is distinctive (Payne and Risley 1976), and it is bio chemically separable from its closest relatives in the genus Egretta (Sheldon 1987, Sheldon and Whittingham 1997).
Range and status
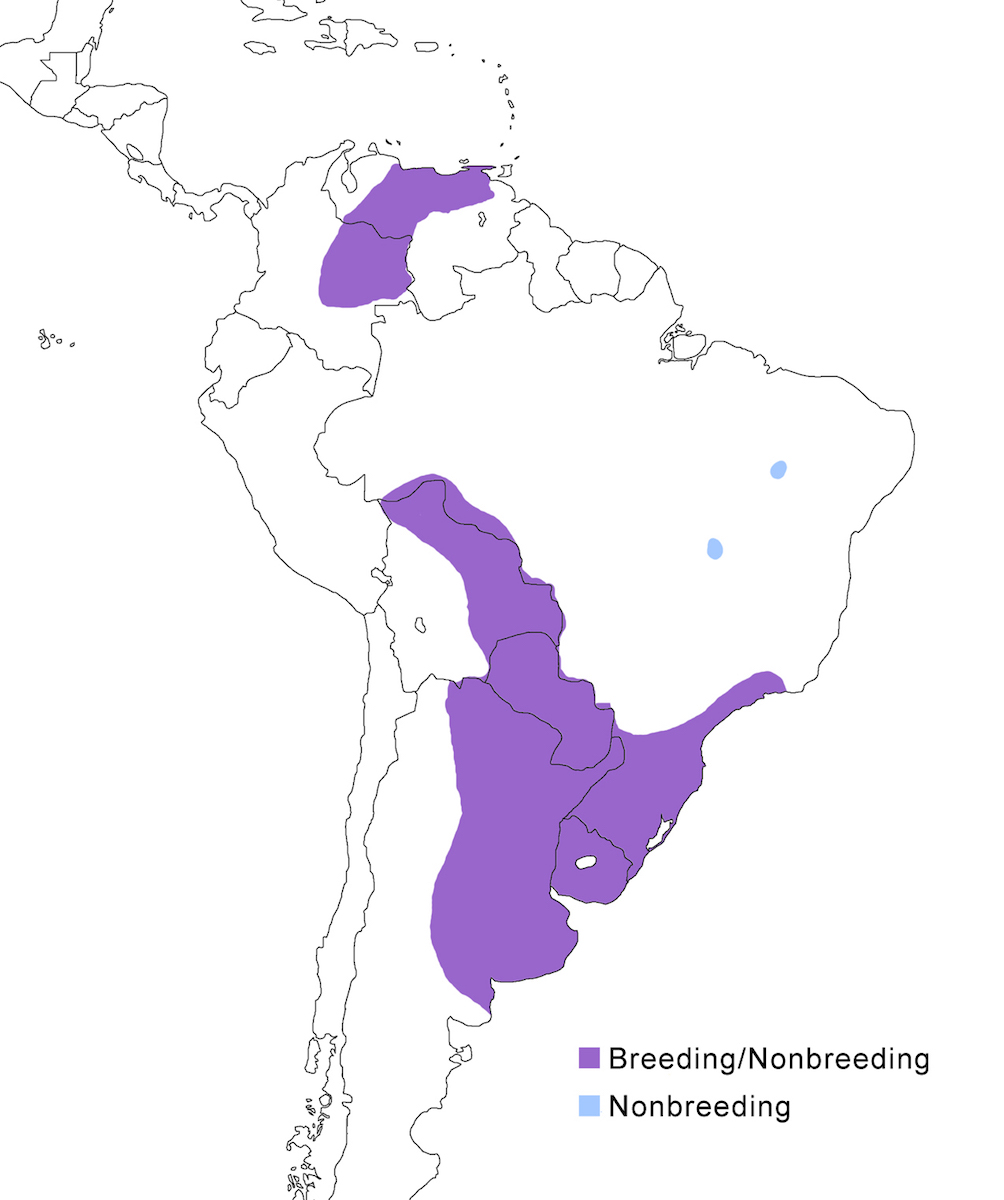
The Whistling Heron is a South American endemic.
Breeding range: It has two disjunct populations in the South American lowlands, apparently at least partially separated by the Amazon basin. The northern subspecies fostersmithi occurs in northern South America east of the Andes in the Llanos and Orinoco basin in Colombia (south to Meta and Arauca - although there are not yet any breeding records from Colombia) and Venezuela (Barinas, Apure east to Monagas and northern Bolívar). The southern race, sibilatrix, occurs in Bolivia (Beni, Santa Cruz, Tarija), Paraguay and Brazil (Mato Grasso, Sao Paulo, Uruguay, Bolivia, Argentina (Tucumá, Sante Fe, Buenos Aires) and Chile (Blake 1977, Antas et al. 1986, Sibley and Monroe 1990, Blanco and Canevari 1992, Sick 1993). The species is expanding its range in southern South America, including into Chile, southwards in Argentina, and northward in Brazil (Morales 2000). A recent sighting was in northeast Brazil, well outside its expected range (Olmos in Morales 2000).
Migration: It is possible that the species undergoes seasonal migrations and population shifts, but more information is needed for both populations. Such shifts are likely in response to wet/dry cycles. It is migratory in northeast Venezuela, being absent from November to January (Friedmann and Smith 1950). In the south, it appears to move in response to droughts but is found in some areas (e.g., Buenos Aires) throughout the year. A dispersal record is in east Brazil (Piaui) , well outside its known range (Morales pers. comm.).
Status: The species is locally common to very common in appropriate habitat and common seasonally in the Llanos. It is, however, often patchily distributed in response to habitat conditions. Amerindians have been recorded as using its plumes for barter, but not sufficiently to cause significant pressure on numbers. It is not endangered anywhere in its range.
Habitat
This is a heron characteristic of the open seasonally flooded savannah (Kushlan et al. 1982).Although very typically found in dry grassland and fields, like other “dry land” herons it more typically uses damp and very shallowly flooded situations such as prairies wet after rains, stream edges, ditches, ponds, pools, lake shores, flooded woodlands, and rice fields. It uses man-altered habitats such as managed pastures and fields, but also road edges and ditches. It uses tree stands for roosting and nesting and is therefore something of an ecotonal species preferring an intermixture both grassland and woodlot.
Foraging
Whistling Herons forage simply, typically by Standing or Walking, usually with the head held high and the arched neck in an erect S (Kushlan et al. 1982, Hancock 1999). They hold the bill horizontal or Peer Over or point the head downward staring at a likely strike spot for many minutes. Strikes commonly follow slowly lowering the head before a Bill Thrust or Pecking on wet ground, Herons sometime use Neck Swaying before a strike. They also Walk Slowly scanning the feeding area and can Run after prey in a Crouched posture. Whistling Herons are ready to catch anything suitable that happens their way. They use Standing Flycatching, Gleaning from plants, and even attempted piracy when the opportunity arises in the course of foraging.
Whistling Herons typically feed alone or in pairs in defended feeding territories (Kushlan et al. 1982). Feeding territories are defended by bouts of ground displays, including Crest Raising, Forward displays, Stretch displays, whistles, and jabbing and probing opponents with their bill. A bird appears reluctant to abandon its feeding site, such as a roadway even when pressed by humans. They do sometimes feed in larger groups, numbering in the dozens, often prior to roosting.
They perch on fence posts or in trees within or near the feeding area. They also roost in trees and, despite feeding dispersed, in the evening they may assemble in arboreal roosts that can number in the hundreds. Arriving about an hour or longer before dark, they become highly social, call loudly with raised crests, preen, spar and aggress on each other.
Food taken is varied, depending no doubt on seasonal availability. As is typical of dry land herons, the diet is dominated by arthropods of all sorts, including spiders, beetles and beetle larvae, dragonfly larvae, flying insects, mantis, and also can include earthworms, eels, frogs, tadpoles, glass snakes (Ophiodes lizards).
Breeding
The breeding season appears to be very extended, and variable between and even within populations. Breeding is April to September in the north, September to January in Brazil; January in Uruguay.
The Whistling Heron nests in mature trees, such as araucarias or even exotic species, especially in small wood lots and forest remnants (Olmos 1989, Anjos 1990). They nest singly or in small, scattered colonies, traditionally returning to previous nest locations form one year to the next. The nest is a loosely built platform of twigs, usually placed on a thick horizontal branch situated high in the tree, 3 to 11 m.
Very little has been recorded about this heron’s display, but it apparently quite often adopts the bitterning posture (Kerr 1950). There is an aerial display involving flying back and forth and gliding in circles. Payne observed a captive pair raising their long black crest plumes in display (Payne and Risley 1976). The normal clutch is 3 or 4 eggs. Eggs are pale blue and speckled, measuring 46-48 x 35-37 mm. Incubation period is typical of herons, about 28 days (Di Giacomo 1988).
Very little is known about chick growth and development. Di Giacomo (1988) reported the nestling period to be 42 days. Adults and young maintain post-fledging associations for some months; how they dissolve is not reported. They communicate by whistles and hissing. Young birds are fed by their parents after fledging. Young beg by hissing and drooping their wings (Hancock and Kushlan 1984). In exotic trees, nests were lost in storms; egg survival was 28% and nestling survival was 40% (Di Giacomo 1988). He found an average production of only 0.29 fledglings per nest. However, based on observations of young with adults in more natural habitat, it appears that two young normally fledge.
Conservation
Where it occurs, the species is common to very common and expanding its range. As a bird adapted to grassland benefiting from forest conversion, rice growing, and the expansion of cattle ranching, so long as the system is not overly dried and trees and woods for roosting remain. They are frequently seen in human-altered environments, even industrial sites (Olmos 1989). Based on available information, there is not a need for specific conservation planning, other than the protection of flooded grasslands on which they depend. Habitat suitability may depend on maintenance of naturally occurring seasonal fluctuations in water levels and soil moisture and, given the species’ dependence on interspersed grassland and trees, complete conversion of forests to pasture would adversely affect it.
Research needs
In many ways, this is a very little known species. Neither its nesting biology nor the variability and seasonality of feeding is well understood. Its population biology is unknown, and could be very instructive given the un-heron-like prolonged dependence of young on adults. It would be intriguing to determine the relatedness among birds occupying the same area and how birds enter the local breeding population. Mortality, survivorship, and dispersal, need to be understood. The study of visibly marked individuals over multiple years could be of significant value in understanding this species. Given the few specimens studied and their tendency to fade with time, a restudy of the racial distinctiveness of the two populations, based on additional fresh specimens, is needed. This is of conservation importance given the recent observations well outside the accepted range (Morales 2000).
Overview
The Whistling Heron remains an enigmatic species. It is a bird of dry to damp grasslands, like several other herons, and so has specialized morphology to accommodate a semi-terrestrial way of life. It Walks and Stands, as would be expected of a grassland heron, and takes what it can capture. However, its ecology is rather distinctive, being sedentary and appearing to maintain family groups. Its calls and exuberant behaviour at the roost are unusual among herons. Furthermore, the prolonged dependence of young on adults is decidedly unheron-like. Explanations may be the need to teach foraging, but such dependence in other species suggests a saturated habitat and maybe young birds face difficulty in establishing their own territory. Given the difference of this family life to that of most herons, the species is likely to also show unique demography and social structure. It is reported that some local people do not think of the species as a heron, so it likely is more biologically interesting than is now known.