Monitoring heron populations in Italy, 1972-2010
Abstract
In this paper, we describe a 39-year long monitoring of heronries in Italy, providing an informative case history for such a long term monitoring program. Initiated in 1972, the program is still running covering 57,600 km2 in NW Italy. The survey area is one in which colonial Ardeids are particularly abundant due to large areas of rice cultivation. The censuses were performed by teams of collaborators operating under centralized coordination, using the technique best suited to the condition of each heronry. The techniques used were: ground count of all nests; post-nesting ground count; aerial count; and perimeter count. An annual population index and index trend were computed using the TRIM index. All the 7 species underwent large but gradual changes in population size during the 39 years of monitoring. In pace with population increase, the number of heronries increased from 40 in 1972 to 175 in 2010. Heron populations increased from 1980 to 2000, after which Grey Herons, Little Egrets, and possibly Squacco Herons leveled off with population levels 3 to 20 times higher than their initial levels. Black-crowned Night Herons initially increased like the other species but later reversed its trend. These heron populations appear to have been sensitive to environmental and climatic changes, temporal variation in human disturbance, and changes in foraging habitats, with these elements differing in importance for each heron species. Reduced human-induced mortality likely is the main cause triggering the increases. We evaluate the critical features of our monitoring program, including its casual onset and later developments, opportunity to witness significant population changes, goal definition, limitations in sampling procedures, factors affecting population change, and cost effectiveness. Our experiences may be used to inform the development of long-term monitoring programs elsewhere.
Key words: Ardea; Ardeola; climate change; conservation; Egretta; hunting mortality; long-term monitoring; Nycticorax; population trends; rice fields.
Introduction
We conducted a large-scale, long-term monitoring program of the breeding colonial Ardeids throughout Northwestern Italy from 1972 to present. From a conservation perspective, monitoring of these populations was critical as the region holds heron populations of continental importance, including up to 15% of the European total for Little Egrets (Egretta garzetta) and Black-crowned Night Herons (Nycticorax nycticorax) (Fasola and Ruiz 1996a, 1996b). Grey Herons (Ardea cinerea), Purple Herons (A. purpurea), Little Egrets, Squacco Herons (Ardeola ralloides), and Black-crowned Night Herons bred throughout the entire monitoring period, whereas Cattle Egrets (Ardea ibis) and the Great White Egrets (Ardea alba) started breeding during the monitoring period.
During our monitoring period, the Ardeid populations underwent notable changes and a general increase. These increases are attributable to several factors, as discussed elsewhere for the period 1972-2006 (Fasola et al. 2010). Here, we update the description of the population trends, and we discuss the the monitoring program, including both achievements and shortcomings, as a case history to inform similar long term monitoring programs.
Methods
The censuses were performed initially by a small team, but after 1981 when the number of heronries started to increase, they were performed by teams of ornithologists, students, park wardens. A centralized database was maintained at the Dipartimento di Biologia Animale, Pavia University. Standardized instructions were provided to the collaborators, and training sessions in the fields were held annually in order to homogenize the counts. Although we could not evaluate the differences in nest counts of our collaborators, whose accuracy could have been low (Graham et al. 1996), the censuses were performed by the same teams for several years and for the whole study period in some case. Therefore we are confident the censuses were reasonably precise, i.e. repeatable over the years, and therefore suitable for the calculation of population trends. All the techniques for censusing colonial waterbirds have insurmountable shortcomings (Kushlan 1992). Different techniques provide estimates with large confidence intervals (>20%, Dodd and Murphy 1995). Accuracy is weakened by incomplete synchrony of breeding (Frederick et al. 2006), and by short nest persistence (Piazza and Wright 2004), so that no practicable technique can count all the nests that existed throughout an entire breeding season. Despite all our efforts to standardize the techniques adopted for our monitoring, the counts can only be interpreted as indicators of nest numbers at peak breeding. Within these limits, however, we believe that the number of nest recorded during our counts, albeit of unknown accuracy, can provide a sufficiently precise index of the long-term temporal changes in the abundance of breeders.
Our collaborators were asked to visit each heronry at least two times during the breeding season, and to provide an estimate of the total number of nests at the peak of colony occupation, using the technique best suited to the condition of each particular heronry. Counters used one of the following techniques (Dodd and Murphy 1995):
1) Ground count of all the nests. This technique was used for 66.4% of the heronries throughout our monitoring, and particularly to the easily accessible, small and monospecific heronries, where birds were not severely disturbed by observers.
2) Post-nesting ground count. This is a technique advocated by Gibbs et al. (1988). During breeding, the proportion of each species was estimated on a sample of >50 nests during >2 visits. These proportions were then extrapolated to the total number of nests, counted during the next winter on leafless trees. Grey Heron nests, which were large, were readily distinguished during post- breeding counts. The number of nests at winter count was multiplied by a conversion factor (1.12 for the Grey Heron, and 1.06 for the other species) to account for the average number of nests that disappear from breeding to winter. These conversion factors had been estimated as the average ratio between total counts performed twice, during breeding and during the subsequent fall, at 20 sample heronries (M. Fasola, unpublished data). Post-breeding nest counts were used at 32.6% of the heronries, particularly for large, mixed colonies, and when disturbance of a complete count during breeding was not advisable. Even in these heronries, however, the scarce species, such as Purple and Squacco herons, Cattle and Great White egrets, were counted individually during breeding, because their estimated proportion would be far less accurate than for the abundant species.
3) Aerial count. This technique is a count of nests on low-altitude photos, used particularly for the few cases (0.4%) of purple heron colonies in reed beds. 4) Perimeter count. This technique is based on expert estimates of visible nests and foraging flights, observed from the colony edge. It was used in only a few cases (0.6%) for small and inaccessible heronries, where other techniques could not be applied.
A single census technique was usually used year after year for the same colony. The few nests that may have remained in abandoned portions of the heronry from previous years were identified during breeding season and not counted. Within the active portion of the heronry, on the other hand, we did not ascertain whether a nest had been used during the present breeding season, because observations of large samples of marked nests (Fasola 1998), showed that in active heronries, unguarded nests disappear in a few days because neighbor breeders rapidly remove their twigs.
The counts were performed from late April to early June at the peak of the breeding season, usually synchronized among species, with the exception of Grey Herons that bred earlier and were counted in April. Until 1980, an average of 34.3% of the heronries were censused each year, while since 1982 a larger sample was censused (66.3%), and complete censuses were performed in 1981, 1986 and 2002. For the heronries not censused in a given year, breeding was confirmed for each species, thus providing the information necessary for the calculation of a population index.
Incomplete counts such as our monitoring, are typical of large-scale censuses of colonial birds (e.g., the Grey Heron in the UK; Marchant et al. 2004), and require the use of population indexes rather than raw counts. We computed the annual population of ardeids using the index implemented by the TRIM software (Pannekoek and van Strien 2001), based on a loglinear Poisson regression method. This index is expressed as ratio between the population in each given year and that of 2000, assumed as base year. The TRIM software, specifically developed to analyze monitoring data from incomplete counts, which is commonplace in ecological surveys, accounts for overdispersion and temporal autocorrelation of such data (van Strien et al. 2004). We used the general habitat as TRIM categorical covariate (factor that group individual sites on the basis of a feature hypothesized to affect populations) in three categories (Fig. 1): “rice fields” (heronries located in lowlands with abundant rice fields, where all the species bred since the seventies), “rivers” (heronries located in lowlands along rivers, where an increasing number of herons appeared only after 1980), and “upland” (heronries located in areas of higher elevation, from 250 to 650 m above sea level that were colonized only after 1990.
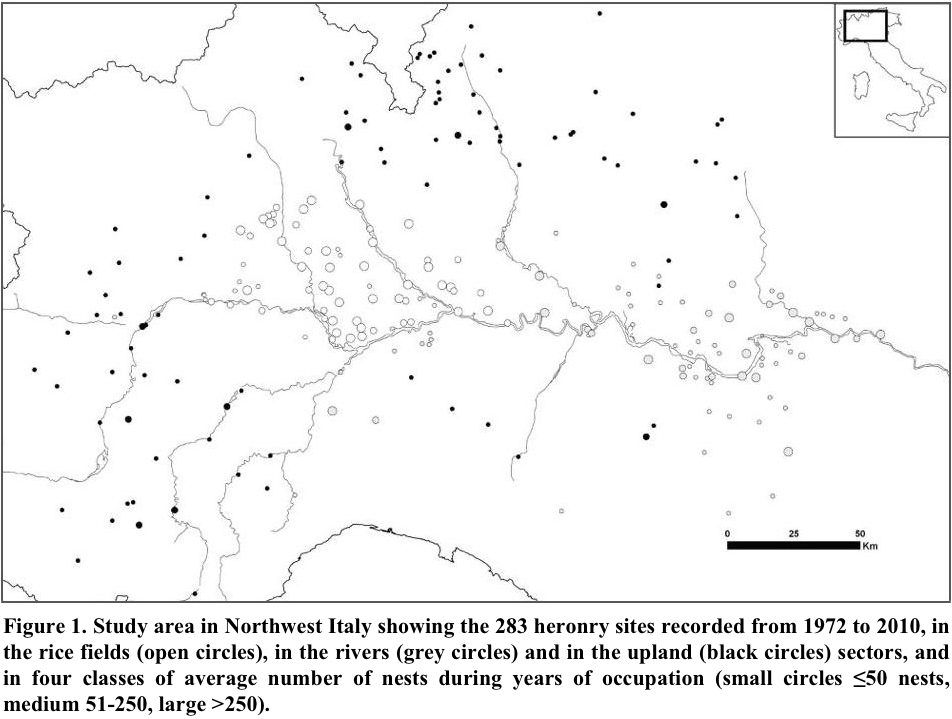
Study Area
The heronries were monitored throughout an area of 57,600 km2 in NW Italy (Fig. 1) where colonies are particularly abundant owing to the presence of the largest extension of rice cultivation within Europe, 2,143 km2 in 2006 (http://www.enterisi.it/servizi/notizie/notizie_homepage.aspx). The heronries are located in small wetlands, with alder woods, bushy willows, and reed beds, or dry mature woodlands, false acacia groves, and poplar plantations among the cultivated and urbanized landscape, and sometimes in wooded parks (Fasola et al. 2007). We defined as one colony each group of nests distant enough from neighbor groups so that birds of the distinct groups did not interact behaviorally, usually at distances >1 km (Buckley and Buckley 1979). Most heron colonies have remained at the same site for long, and some had been recorded at the beginning of the Twentieth century by Moltoni (1936). Other colony sites became inactive during the monitoring period owing to habitat destruction, human disturbance, or unknown causes. Many new heron colonies appeared, in pace with population increase; and they expanded on parts of the study area that had not been occupied previously including rivers and uplands, which likely were less optimal than the traditionally occupied rice fields (Fasola and Brangi 2010). The total number of heron colonies active in each year increased from 40 in 1972 to 175 in 2010. From 1972 to 2010, we recorded 283 heron colonies. Of these, 22 were occupied only temporarily (for <3 years and with <5 nests). The re-occupation of traditional colony sites and the repeated, independent discovery of the same heronries, suggested that all the colonies within the study area had been identified. If nesting was overlooked, they were few and isolated nests.
Results and Discussion
Population trends over four decades.
All the seven heron species underwent large changes during the 39 years of monitoring (Fig. 2). A notably regular trend was shown by Grey Herons, and a lower regularity by some other species. The annual changes by Squacco Herons were the most variable, possibly due to occasional biases in the counts of this less abundant species. All species exhibited directional trends and all overall, except the Black-crowned Night Heron, exhibited a strong increase. Grey Herons increased regularly since 1984 and after 1998 leveled off at about 20 times the mean number in 1976-1978. Little Egrets increased to about four times their 1976-1978 mean. These trends are well described by logistic models (Fasola et al. 2010). Purple Herons and Squacco Herons seem still to be increasing. Black-crowned Night Herons showed a different pattern. During the initial part of monitoring, they increased but after peaking in 1989, they decreased that after 2004 remained apparently stable. The two new colonizers, Cattle and Great White egrets, appeared around 1990 and showed trends that seem to fit the initial phase of a logistic expansion, a pattern typical for species expanding into a new region (Kushlan and Hancock 2005).
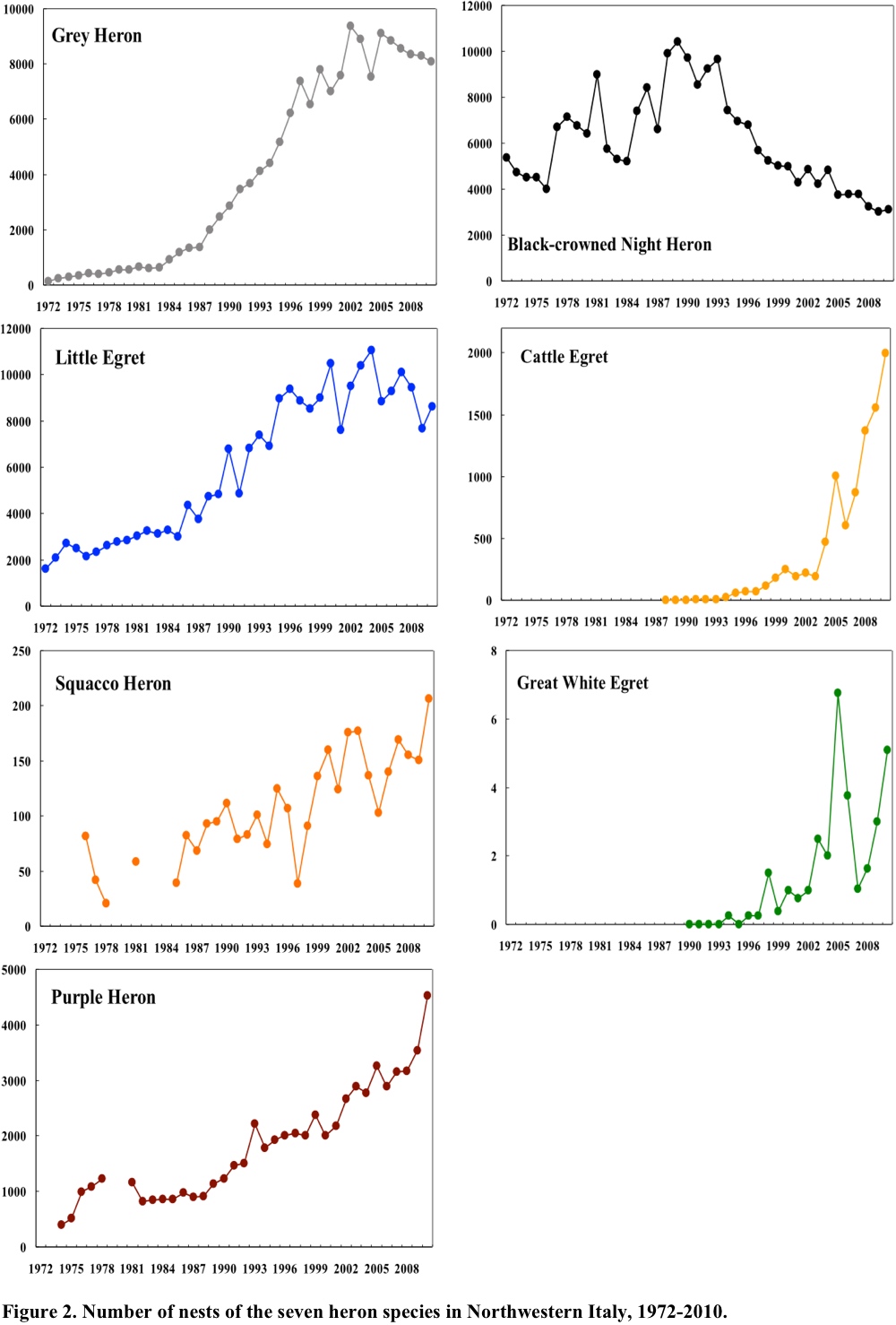
In summary, the trends in population change during the past 39 years were strikingly smooth and directional. A period of strong increase lasted from 1980 to 2000, after which Grey Herons, Little Egrets, and possibly Squacco Herons leveled off with population levels 3 to 20 times higher than the initial level. The Black-crowned Night Heron initially increased like the other species, but later reversed its trend.
The factors that may have affected such large population changes have been assessed using Auto-Regressive Integrated Moving Average (ARIMA) models (Fasola et al. 2010). Among several candidate ecological factors, significant effects on Grey Herons were the decreased human-induced mortality, as quantified by an index of hunting pressure, and by increasing winter temperatures. Significant effects on Little Egrets were increased extent of rice fields. Significant effects on Squacco Herons were increased rainfall in their African wintering range. Black-crowned Night Herons were positively affected by increasing African rainfall during 1972-1988. The decrease of Night Herons, which contrasts sharply with the trend of all the other species, may be related to competition with other, increasing, herons. The hypothesis that Black-crowned Night Herons could have been limited by the strong increase of the larger and competitively superior Grey Heron, is somewhat suggested by their niche overlap. Black-crowned Night and Grey herons overlap in prey type and size and in foraging habitats, more than any of the other species of the Italian herons and egrets (Fasola 1986, 1994). But the peculiar trend of Black-crowned Night Herons could also depend on other, poorly known factors, such as the climate in the overwintering areas that could partially differ from the areas of the other migratory herons.
The heronry sites of NW Italy enjoy a satisfactory level of protection owing to the enforcement of site-specific conservation actions for several of the colony sites (Fasola and Alieri 1992). The improved protection of colony sites within special reserves was also considered as a factor affecting populations (Fasola et al. 2010), but it was found unlikely as primary trigger of the observed increase, although obviously important for the long- term population persistence.
The heron populations in NW Italy are sensitive to environmental and climatic changes, as well as to temporal variation in human disturbance and changes in foraging habitats, with differing importance of each factors for each heron or egret species. The decreased human-induced mortality could have been the main cause triggering the increases. In the early 20th century, several European heron populations were at a minimum, due to persecution as pests for freshwater fisheries and as game species, but many subsequently recovered (Marion 1997; Kushlan and Hafner 2000; Kushlan and Hancock 2005).
Critical features of the monitoring program.
Long-term studies, i.e. those lasting more than the longevity of the study species and thus including some generation turnover, are the indispensable tool to resolve population phenomena (Cody and Smallwood 1996). For large birds, such as herons with the long generation time, such studies have to encompass decades, and in this case, accurate planning and cost-effectiveness are of great concern (Goldsmith 1991, Thompson et al. 1998). Our study is a case history in such a monitoring program, but one that may have value in informing the development of long term programs. Several important considerations were as follows.
Casual onset and later developments. The program was unplanned. The monitoring program of heronries in Italy started as individual under-graduate research when colony location was sought as information necessary for further studies on reproductive biology. It then expanded into regional survey of all the heronries, and eventually continued as coordinated monitoring.
Opportunity to witness significant population changes. This monitoring program, albeit not prompted by any expectation of population change, was fortunate enough to include a period of notable and directional change in population size of all species and was able to document this change.
Goal definition. Despite a lack of explicit initial goal, this program accomplished several ends by providing: basic information needed for further studies on foraging and breeding biology; initial information for the creation of nature reserves that conserve the heronries themselves and with them also the wetland biotopes within the urbanized landscape of Northern Italy; information crucial for the planning and management of the nature reserves; and a data base for population analysis.
Limitations in sampling procedures. As a consequence of the casual start, this program could not be designed from the beginning, therefore there were shortcomings, especially during the first years, in the techniques (e.g. lack of tests for inter-observer differences in the counts), and in the collection of information on variables critical for the interpretation of population changes (among which, on foraging ecology, reproductive rate).
Factors affecting population change. After the first years of heronry monitoring, hypotheses surged among the participants and others on the factors driving the observed changes, and collateral studies were initiated on such factors, including colony site availability (Fasola and Alieri 1992), foraging ecology and prey density in rice fields (Fasola 1986, 1994), reproductive success (Fasola 1998), and pesticide residues (Fasola et al. 1998). These factors however were surveyed in a sample of a few years when funding was available, but could not be assessed at regular intervals, and have not been studied after 2000. Such missing information is the main shortcoming of our program, because population dynamics cannot be fully explained unless the underlying demographic mechanisms are known. Any monitoring program concerned with the factors driving population variations, should include records of productivity, survival, and proportion of breeders, all of which are essential for the understanding of population regulation of birds (Newton 1998).
Costs. Monitoring can entail large costs when repeated over long time and a large area. This program on heronries in Italy is carried out mainly through voluntary collaboration, with minimal costs for the census of the heronries non covered by any collaborator, for coordination and for data management, and can be considered highly cost-effective. Thus it seems that monitoring need not be expensive.
The monitoring program described in this paper provided substantive information on the changes in heron populations in a region of Italy. That it began unplanned and counting techniques varied yet documented critical information on population changes is encouraging. It is hoped that the experiences and lessons learned in this program can be of value in the development of heron monitoring programs elsewhere.
Acknowledgements
We are deeply indebted to all the collaborators of the research group Garzaie Italia who performed the censuses at the colonies, recently Gianfranco Alessandria, Marco Baietto, Marco Bandini, Angelo Battaglia, Giovanni Bazzano, Luigi Beraudo Franco Bernini, Giovanni Boano, Anna Bonardi, Gian Abele Bonicelli, Piero Bonvicini, Alberto Boto, Anna Brangi, Sandra Buzio, Monica Carabella, Franco Carpegna, Pietro Cassone, Bruno Caula, Francesco Cecere, Mauro Della Toffola, Flavio Ferlini, Diego Ferri, Alessandra Gagliardi, Arturo Gargioni, Claudio Gioda, Laura Gola, Nunzio Grattini, Walter Guenzani, Franco Lavezzi, Daniele Longhi, Violetta Longoni, Lorenzo Maffezzoli, Edoardo Manfredini, Valentina Mangini, Cesare Martignoni, Fabrizio Nobili, Giuliana Pirotta, Cristina Poma, Ivan Provini, Daniele Reteuna, Alessandro Re, Bassano Riboni, Ettore Rigamonti, Domenico Rosselli, Walter Sassi, Fabrizio Scelsi, Alberto Tamietti, Eugenio Tiso, Andrea Viganò, Enrico Viganò, Alfredo Zambelli, and many others for the past years. The Regione Lombardia supported the program. Nicola Gilio helped with the maps. Our remembrance goes to the late friends Heinz Hafner and Francesco Barbieri with whom we shared so many years of fieldwork and discussion.
Literature Cited
Buckley, P. A. and F. G. Buckley. 1979. What constitutes a waterbird colony? Reflections from the northeastern U.S. Proceedings of the Colonial Waterbirds Group 3:1-15.
Cody, M. L. and J. A. Smallwood. 1996. Long-term Studies of Vertebrate Communities. Academic Press, San Diego, U.S.A.
Dodd, M .G. and T. M. Murphy1995. Accuracy and precision of techniques for counting Great Blue Heron nests. Journal Wildlife Management 59:667-673.
Fasola, M. 1986. Resource use of foraging herons in agricultural and nonagricultural habitats in Italy. Colonial Waterbirds 9:139-148.
Fasola, M. 1994. Opportunistic use of foraging resources by heron communites in Southern Europe. Ecography 17:113-123.
Fasola, M. 1998. Optimal clutch size in herons: observational and experimental approaches. Ethology Ecology Evolution 10:33-46.
Fasola, M. and X. Ruiz. 1996a.The value of rice fields as substitutes for natural wetlands for waterbirds in the Mediterranean Region. Colonial Waterbirds 19 (Special publication 1):122-128.
Fasola, M. and X. Ruiz. 1996b. Rice farming and waterbirds: integrated management in an artificial landscape. Pp. 210-235, In: Farming and Birds in Europe: The Common Agricultural Policy and its Implication for Bird Conservation (D. J. Pain and M. W. Pienkowski, Eds.). Academic Press, London, U.K.
Fasola, M., P. Movalli and C. Gandini. 1998. Heavy metal, organocholorine pescidide, and PCB residues in eggs and feathers of herons breeding in northern Italy. Archives Environmental Contamination Toxicology 34:87-93.
Fasola, M. and R. Alieri. 1992. Conservation of heronry sites in North Italian agricultural landscapes. Biological Conservation 62:219-228.
Fasola, M., G. Albanese, ASOER, G. Boano, E. Boncompagni, U. Bressan, M. Brunelli, A. Ciaccio, G. Floris, M. Grussu, R. Guglielmi, C. Guzzon, F. Mezzavilla, G. Paesani, A. Sacchetti, M. Sanna, F. Scarton, C. Scoccianti, P. Utmar, G. Vaschetti and F. Velatta F. 2007. Le garzaie in Italia, 2002. Avocetta 31:5-46.
Fasola, M. and A. Brangi. 2010. Consequences of rice agriculture for waterbirds population size and dynamics. Waterbirds 33 (Special Publication 1): 160-166.
Fasola, M., D. Rubolini, E. Merli, E. Boncompagni and U. Bressan. 2010. Long- term trends of heron and egret populations in Italy, and the effects of climate, human- induced mortality, and habitat on population dynamics. Population Ecology 52:59-72.
Frederick, P. C., J. A. Heat, R. Bennets and H. Hafner. 2006. Estimating nests not present at the time of breeding surveys: an important consideration in assessing nesting populations. Journal Field Ornithology 77:212-219.
Gibbs, J. P., S. Woodward, M. L. Hunter and A. E. Hutchinson. 1988. Comparison of techniques for censusing Great Blue Heron nests. Journal Field Ornithology 59:130-134.
Goldsmith, F.B. 1991. Monitoring for conservation and ecology. Champan & Hall, London, U.K.
Graham, K., B. Collier, M. Broadstreet and B. Collins. 1996. Great Blue Heron (Ardea herodias) population in Ontario: data from and insight on the use of volunteers. Colonial Waterbirds 19:39-44.
Kushlan, J. A. 1992. Population biology and conservation of colonial wading birds. Colonial Waterbirds 15:1-7.
Kushlan, J. A. and H. Hafner. 2000. Heron Conservation. Academic Press, San Diego, U.S.A.
Kushlan, J. A. and J. A. Hancock. 2005. The Herons. Oxford University Press, Oxford, U.K.
Marchant, J. H., S. N. Freeman, H. Q. P Crick and L. P. Beaven. 2004. The BTO heronries census of England and Wales 1928-2000: new indexes and a comparison of analytical methods. Ibis 146:323-334.
Marion, L. 1997. Évolution des effectifs nicheurs et de la répartition des hérons coloniaux en France entre 1974 et 1994. Alauda 65:86-88.
Moltoni, E. 1936. Le garzaie in Italia. Rivista Italiana Ornitologia 6:109-148; 211-296.
Newton, I, 1998, Population Limitation in Birds. Academic Press, London, U.K.
Pannekoek, J. and A. van Strien. 2001. TRIM 3 Manual (Trends and Indexes for Monitoring data). Statistics Netherlands, Voorburg, The Netherlands.
Piazza, B. P. and V. L. Wright. 2004. Within-season nest persistence in large wading bird rookeries. Waterbirds 27:362-367.
Thompson, W. L., G. C. White and C. Gowan. 1998. Monitoring Vertebrate Populations. Academic Press, San Diego, U.S.A.
van Strien, A., J. Pannekoek, W. Hagemeijer and T. Verstrael. 2004. A loglinear Poisson regression method to analyse bird monitoring data. Bird Census News 13:33-39.



