Nest predation of the critically endangered White-bellied Heron Ardea insignis by Masked Palm Civet Paguma larvata in Burichhu, Bhutan #
Abstract
The White-bellied Heron (Ardea insignis) is verging on extinction, but very little is known about its basic ecology and biology, hindering effective implementation of the conservation actions. To date, nothing has been known about the predators of this species, nor the causes of nesting failure sufficiently understood. We carried out a systematic survey to locate the nests of the White-bellied Heron along the Punatsangchhu river basin. One active nest was monitored continuously until the fate of the nest was confirmed. Camera traps were set up on the failed and abandoned nest and on artificial nests baited with dyed chicken and dummy eggs. The evidence strongly suggests that Masked Palm Civet Paguma larvata is an egg predator of the White-bellied Heron and responsible for a single nest failure at Burichhu, Tsirang. Also, potential bird egg predators of at least seven genera were documented. Nest predation bears immense significance in the conservation of rare species. To this end, conservation management should implement evidence-based nest protection methods from their natural predators to reduce and prevent further nesting failures. Additionally, an intensive study is required to glean vital information on the causes of nesting failures, including the nesting predation from their breeding sites and its impacts on their nesting behavior.
Key words: Bhutan, conservation, Masked Palm Civet, nesting failure, nest predation, White-bellied Heron.
# This paper was presented at the virtual Symposium on Herons of World-wide Conservation Concern held on 9 November 2021.
Introduction
The White-bellied Heron (or WBH) Ardea insignis is critically endangered with an estimated global population of 50-249 mature individuals (BirdLife International 2018) while only 60 individuals were recorded across their range countries (Price and Goodman 2015). The population distribution of WBH seems to have undergone further contraction from its once common areas in Bhutan, India and Myanmar, while extirpated from Nepal and Bangladesh (BirdLife International 2001). The WBH is described as ‘solitary, very wild and wary’ (Baker 1929).
The first active nest of WBH was reported from Bhutan in Zawa, Wangduephodrang district in 2003 (Royal Society for Protection of Nature [RSPN] 2011), nearly 70 years after its last record from Myanmar. While nesting in Bhutan has exclusively occurred in the Chir Pine Pinus roxburghii since its first sighting, the first successful breeding on the broad-leaf species was reported (Khandu et al. 2020a) in 2018. Limited information available on its breeding behavior suggests that the WBH requires tall trees in an undisturbed forest for its nesting (Baker 1929). WBH preferred tall trees for nesting as they provided open space for their landing and take-off flights while increasing the visibility of the surroundings (Mondal and Maheswaran 2014, Acharja 2019a). The WBH breeds only once a year which normally lasts about five months from the onset of its courtship behavior from February to fledging of the juveniles towards June.
Nest failure in WBH seems quite common if not frequent in Bhutan. In every breeding season, about 1-2 cases of nesting failure from critically low numbers of active nesting counts (ca. 2-4) are reported (S. Tshering, pers. comm.) from Bhutan which is the breeding stronghold in the region. In 2019, a two-week-old chick and two eggs went missing from a nest in Tsaidang, Zhemgang district due to unknown reasons; likewise in 2020, two eggs followed the same fate while a recently hatched chick succumbed to parental infanticide (Acharja et al. 2021). Both Ada and Nangzhina areas under Wangdue Phodrang district also reported nesting failures in the past (RSPN 2009). Records also show that two WBH nests were destroyed by forest fires and one by a windstorm (Acharja 2019a). While there is a lack of in-depth study to understand the actual causes of its nesting failure, it is presumed that nest predators are one of the major factors.
Thus, understanding that nest predation bears immense significance for the conservation of rare species, such as the White-bellied Heron, identifying the nest predator remains challenging. That breeding success directly depends on the availability of food is the accepted paradigm for the international ornithological community (Guppy et al. 2017). It is not until recently that the impact of predation began to be accommodated in the existing paradigm (Birkhead et al. 2014). Nest predation causes reduced nesting success (Ricklefs 1969, Bellamy et al. 2018). Predation risk alone reduced the number of offspring by 40% in the Song Sparrow Melospiza melodia (Zanette et al. 2011). In the absence of mitigation of predation risks, the decline in population persists despite habitat improvement, reduced human disturbances, etc. (McMahon et al. 2020). Therefore, this study presents a record of the first nest predation of WBH causing a single nest failure and provides vital information on other potential nest predators prevalent in their breeding habitat.
Methods and Results
Study Area
This study was conducted in Burichhu (27° 5' 10.15" N, 90° 4' 28.76" E), Tsirang district (Fig. 1). The district is situated at an elevation of 300 to 4,200 m asl (Forest Resources and Management Division [FRMD] 2016). The district generally experiences hot and humid summers and dry and moderately cold winters. The annual rainfall ranges between 1,000-3,000 mm (FRMD 2016) and the temperature ranges from 12°C-21°C (National Center for Hydrology and Meteorology 2017).
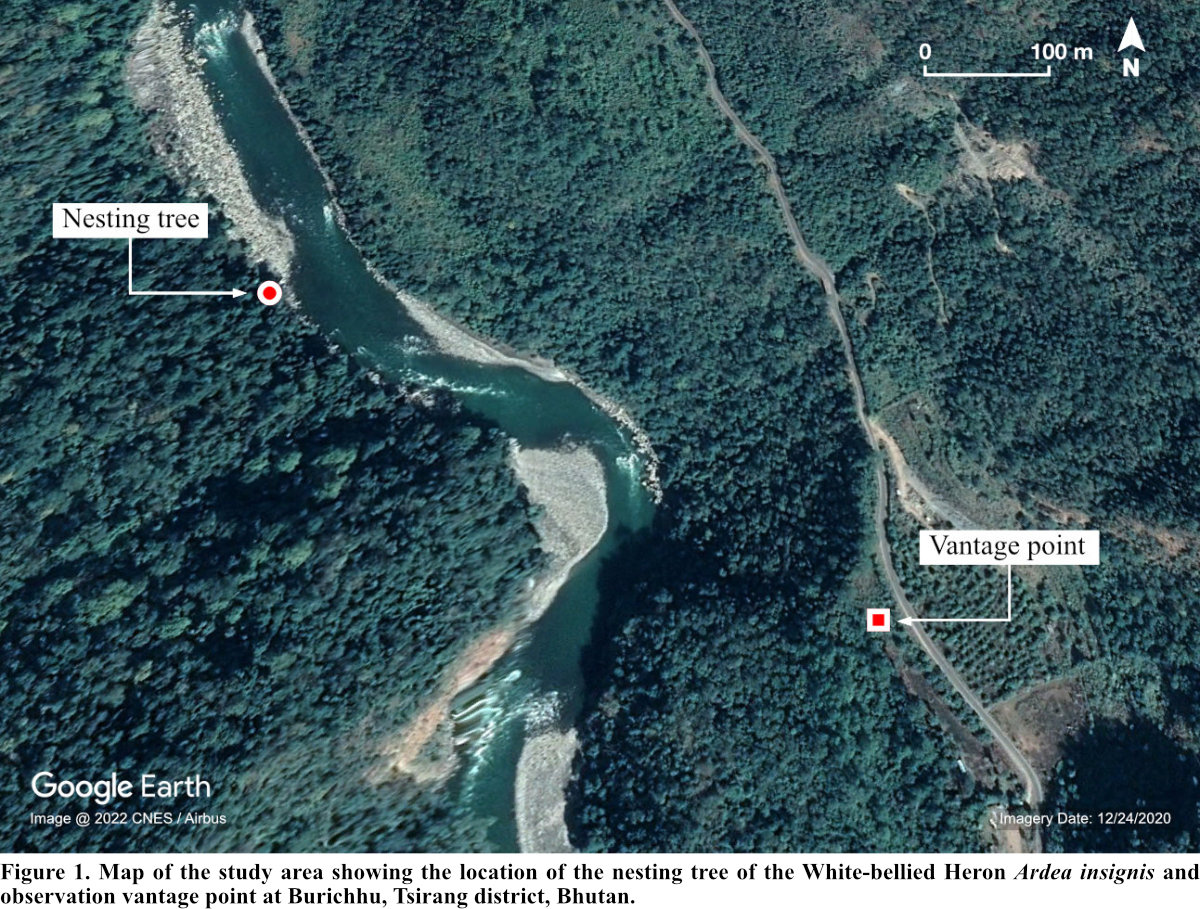
Burichhu is one of the breeding hotspots of the WBH within its habitat in Bhutan with at least five successful breeding records hitherto (Acharja 2019b). WBH is sighted regularly in this region throughout the year owing to the availability of both roosting and foraging microhabitats. The area is sparsely populated by humans with about six households within a 1 km vicinity centroid to our nesting study site. The riverine forest (<370 m asl) is dominated by Tetrameles nudiflora and Syzygium spp. while the mid-region (620-770 m asl) is dominated by Pinus roxburghii (Ghemiray 2016).
Survey and observation of active natural nest
An area count method (Kushlan 2011) with a systematic approach was employed to locate the active nests in the known areas (for details refer to Khandu et al. 2021).
The Punatsangchhu (chhu = river) created a physical barrier between the active nest and the observers, helping reduce disturbance due to our presence. The distance between the observers and the nest was always maintained at ca. 500 m. To address the “uncertainty principle” (Lenington 1979), we made no effort to visit the nest site and take physical measurements during the period of nest occupation by the WBH. Since observer disturbance is associated with reduced nesting success (Götmark 1992) and can cause nest abandonment and desertion (Conover and Miller 1979, White and Thurow 1985), all observations were made vigilantly following necessary nest watching protocols (Philips et al. 2007). Also, our initial observation from the same location appeared to pose no disturbance as inferred through the behavior of the nesting pair.
The continuous monitoring of the active nest lasted for only 11 days (22 May-1 June 2018) because the incubating WBH completely abandoned the nest at 12:58 hr (Bhutan Time; UTC +6 hrs) on the last day. The observations were made from 06:00-19:00 hrs using binoculars (10 x 42) and a 20-60x monocular spotting. The nesting behavior was videoed using a Nikon D7200 coupled with a 500-mm zoom lens with a 2x converter. Our team continued observing the nest until dusk and early morning of the next day hoping for the return of the incubating pair. But the nest was completely abandoned.
Nest examination for the cause of the failure
On 2 June 2018, after almost three hours of journey into the thicket and difficult terrain on foot we reached the nesting site of the WBH. We carefully scanned for any clues such as pug marks, poops, or scratches on the nesting trees and the surrounding areas to discern the fate of the nest. We found pieces of broken eggshells scattered on the ground (Fig. 2A-B) which were separated between 68-223 cm apart. This was a probable clue to rule out that the eggs were not dropped accidentally from the nest. The nest was constructed on the canopy of Magnolia champaca, about 15.5 m in height, 32.8 cm in DBH, and about 150 m from the nearest feeding location.
We climbed up the nesting tree (without any climbing gear) and scanned the open nesting platform (ca. 122 cm in diameter), which was primarily constructed of dry twigs. We found what appeared to be mammalian scats on the nest (Fig. 2C). We collected the scats in a zip lock bag and took their morphometric measurements (Fig. 2D). The scat was mostly composed of intact seeds. The average size of the seed was approximately 1.1 cm in height and 0.8 cm wide.

Camera trapping, artificial nest construction and baiting
After confirming that the nest of the WBH was predated, we set up a camera trap (Ltl Acorn 5310A) on the failed nest along with six chicken eggs dyed in light blue. The camera trap was set up to take one color photo shot per burst in JPEG format (8 megapixels) and videos for 10 sec when the passive infrared sensor was triggered. We recorded two attempts of egg predation from the failed nest by the Masked Palm Civet Paguma larvata (Fig. 3-left).
We also set up six artificial nests following the similar architecture of the natural nests (refer to Acharja 2019a for details) and baited them with 4-6 dyed chicken eggs to capture other potential egg predators within the WBH habitat (Fig. 3-right).

The artificial nests were placed on different live tree species between 100-1,000 m from the natural nest enclosure. All artificial nests were placed with a camera trap (HCO Scoutguard 560C) on the nearest branch from the nest enclosure. Every one to two weeks, the artificial nests were revisited to download the data, replace the camera batteries and refill the baits.
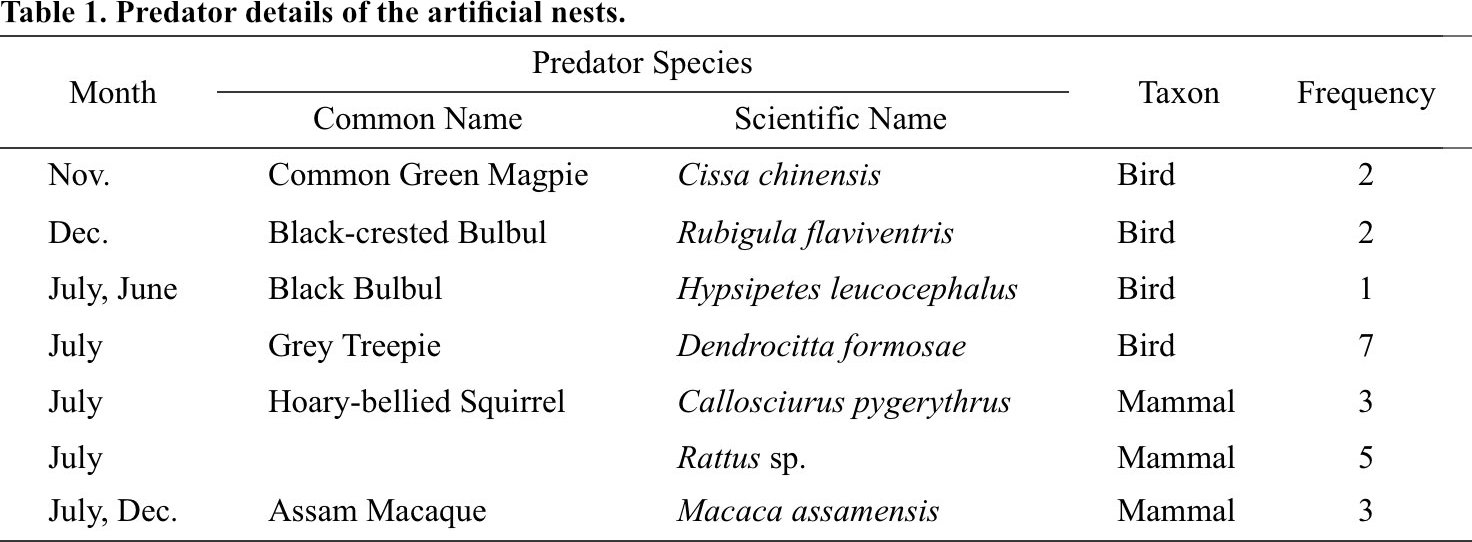
From a total of 76 trapping nights generated from six camera traps over four months, we recorded 7 different species of potential egg predators from the artificial nests (Fig. 4). Grey Treepie Dendrocitta formosae, Rattus sp., Assam Macaque Macaca assamensis and Hoary-bellied Himalayan Squirrel Callosciurus pygerythrus were the predominant predators of the artificial nests while Common Green Magpie Cissa chinensis and Black-crested Bulbul Rubigula flaviventris were sighted twice, and Black Bulbul Hypsipetes leucocephalus was sighted only once (Table 1).

Discussion
Examination of eggshell remnants scattered around the nesting tree and feces on the nest gave us certain information on the identity of the egg predator. However, it was only through the fast deployment of the camera trap with baits on the failed nest that enabled us to capture the most highly likely egg predator. Moreover, civets are also known to repeatedly visit their foraging sites reinforced by the availability of food resources such as the presence of many mature fruits (Zhou et al. 2008). Although no evidence of the nesting predation was recorded until this study, RSPN (2009) speculated that predation by small mammals posits a significant threat to WBH.
The Masked Palm Civet is a generalist feeder with its diet consisting of birds, mammals, reptiles, mollusks, fruits, etc. (Iwama et al. 2017) and shows highly adaptable feeding habits based on the availability of food resources over time and space (Zhou et al. 2008). Therefore, it is possible that nest predation by civets would pose a significant challenge for the breeding success of WBH, particularly when other food sources become scarce or show more preference towards birds and eggs in their diet niche. Landscape modification and habitat loss, particularly forest fragmentation, is also associated with increased predation rates by the generalist predators due to the exposure of concealed nests (Lahti 2001, Eggers et al. 2005). With further loss and degradation of riparian habitat of WBH, mounting anthropogenic pressures such as construction of hydropower and roads, deforestation, sand and gravel mining, forest fires, etc., it is likely that predation risk would become more frequent.
While the Masked Palm Civet attempted twice to predate the egg baits kept in the natural nest of the WBH, it was not captured in any of the artificial nests with the same type of bait and within a similar landscape and habitat. Artificial nests have their limitation to gauge actual predation dynamics by rendering them more attractive than the natural nests for the predators (Bravo et al. 2020) or vice versa. Using dyed chicken eggs might have removed natural egg scent as cues for the potential predators. Likewise, the lack of parental activity at artificial nests could have prevented direct sighting by the predators, causing a decrease in predation rates or, the lack of parent-mediated removal of egg concealment might have the opposite effect (Major and Kendal 1996, Moore and Robinson 2004).
The WBH parents alternately start incubating the eggs as soon as the first egg is laid, without completing the clutch. This behavior further supports the “nest failure hypothesis” which states that birds with higher perceived risk of nest predation early in their nesting cycle favor asynchronous hatching (Clark and Wilson 1981). Also, after hatching we observed that WBH feeds their juveniles alternately in the nest while one of the parents guards the nest consistently. One of the dominant night roosting behaviors of the WBH is resting with open eyes which is possibly related to threat and predator surveillance (Khandu et al. 2020b).
We have also recorded a suite of potential predators belonging to at least seven genera using artificial nests. Since small birds were not much of a direct threat to the WBH as one of the parents guards the nest, it is likely that civets and primates can pose a serious nesting failure. We observed that the presence of a troop of Assam Macaques, Golden Langurs (Trachypithecus geei) and Gray Langurs (Semnopithecus entellus) closer to the nest seemed to alert the parent WBH guarding or incubating. They did not attack or drive the parent WBH away. However, playing, and violent shaking of nesting or neighboring trees by the Macaques and Langurs could potentially cause eggs to drop from the nest.
While the predator removal management approach is highly contentious, its use is a common method to protect the vulnerable bird species and reverse population decline; it is imperative to ascertain its implications based on all available evidence (Smith et al. 2010). A detailed study is required to understand the nest predation of WBH. We found vegetative climbers profusely growing on the nesting tree, which might have provided easy access for the predator to reach up to the nest, which was built on the tree canopy. Removing climbers and shrubs within the periphery of the nesting tree might deter predation attempts and increase nesting success. Going by the mean (± SD) diameter at breast height of the nesting trees (62.0 ± 17.7 cm) (Acharja 2019a), it seems that using nest guards can be effective with fewer resources. The effectiveness of applying various nest guards such as cone and stovepipe baffles has been tested and found to increase the nest success rates by 7% across multiple species (Bailey and Bonter 2017). It has been suggested that the Common Raccoons Procyon lotor are responsible for Grey Herons Ardea cinerea abandoning their nests in Hokkaido, Japan based on claw marks left on the nesting trees (Ikeda 1999, Matsunaga 2005). Additionally, Matsunaga in 2018 observed a raccoon on the nesting tree, probably predating the chicks of the Grey Herons. Consequently, guarding the nest using metal sheets around the trunk of the nesting trees has proved effective as the Grey Herons continued breeding on the same site (Matsunaga 2018). Similarly, the use of predator exclusion called ‘Racoon guards’ were attributed for increasing the nesting success and population of the Great Blue Heron Ardea herodias (Hjertaas 1982, Duyke 2009).
Given our prior investigation of the nesting sites, it is also feasible to erect solar electric fence around the nesting tree to prevent the intrusion of mammalian predators. Electric fencing is a proven technique to prevent nesting predation by small mammals such as coyotes and foxes on the shorebirds (Forster 1975, Winton et al. 2000). Since WBH uses the same nesting trees for multiple breeding seasons, protecting the nesting tree with the help of nest guards and electric fence before the onset of its breeding season would help directly reduce disturbance to the birds and also serve as a long-term investment for the recovery of the WBH population. Empirically tested and viable conservation approaches to nest predation prevention and enhancing nesting success must be explored (more information at Conservation Evidence) and cautiously implemented. Since this study is limited by sample size, extensive further study is required to understand the nest predation rates and enlist the full complement of the nest predators across the WBH habitats in Bhutan and other range countries.
Acknowledgements
I am deeply grateful to Dr. Khursheed A. Khan and wild cat specialist Tashi Dendup for their help in identifying predators from the natural nest. Thanks are also due to Krishna, Sonam Dorji, Sonam Tshering and Chencho Bidha for helping me with the field work. I am forever indebted to Kitichate Shridith, Sara Bumrungsri, George A. Gale, M. Clay Green, Chip Weseloh and Satoshi Shimano for the inspiration to work towards understanding and saving this imperiled yet truly majestic heron species. My deepest gratitude to Katsutoshi Matsunaga and Chip Weseloh for their rigorous review and invaluable feedback for the improvement of this manuscript. I extend my sincere thanks to Department of Forest and Park Services of Bhutan, Tsirang Forest Division, Ugyen Wangchuck Institute for Conservation and Environment Research, Bumthang, for kind support and guidance. I am also indebted to the Nature Conservation Division, Thimphu and Royal Society for Protection of Nature, Thimphu, for loaning camera traps and rendering all possible support.
Literature Cited
Acharja, I. P. 2019a. Evaluation of nest habitat, site preferences and architecture of the critically endangered White-bellied Heron Ardea insignis in Bhutan. Bird Conservation International 30: 599-617.
Acharja, I. P. 2019b. The current population, distribution, and conservation status of the critically endangered White-bellied Heron (Ardea insignis) in Bhutan. Tropical Resources 38: 1-10.
Acharja, I. P., S. Tshering, T. Lhendup and T. Phuntsho. 2021. First observation of sexual conflict and parental infanticide in the Critically Endangered White-bellied Heron Ardea insignis. BirdingASIA 35: 38-42.
Bailey, R. L. and D. N. Bonter. 2017. Predator guards on nest boxes improve nesting success of birds. Wildlife Society Bulletin 41: 434-441.
Baker, E. C. S. 1929. The fauna of British India, including Ceylon and Burma, 2nd edition. Taylor and Francis, London, U.K.
Bellamy, P. E., M. D. Burgess, J. W. Mallord, A. Cristinacce, C. J. Orsman, T. Davis, P. V. Grice and E. C. Charman. 2018. Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. Journal of Ornithology 159: 493-506.
BirdLife International. 2001. Threatened birds of Asia: the BirdLife International Red Data Book. BirdLife International, Cambridge, U.K.
BirdLife International. 2018. Ardea insignis. The IUCN Red List of threatened species 2018. [online]. Accessed 16 June 2022.
Birkhead, T., J. Wimpenny and B. Montgomerie. 2014. Ten thousand birds. Ornithology since Darwin. Princeton University Press, New Jersey, U.S.A.
Bravo, C., O. Pays, M. Sarasa and V. Bretagnolle. 2020. Revisiting an old question: which predators eat eggs of ground-nesting birds in farmland landscapes? Science of Total Environment 744: 140895.
Clark, A. B. and D. S. Wilson. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Quarterly Review of Biology 56: 253-277.
Conover, M. R. and D. E. Miller. 1979. Reaction of Ring-Billed Gulls to predators and human disturbances at their breeding colonies. Proceedings of the Colonial Waterbird Group 2: 41-47.
Duyke, A. L. V. 2009. Conservation and restoration of a Great Blue Heron breeding colony in east central Minnesota. MSc thesis, the University of Minnesota, Minnesota, U.S.A.
Eggers, S., M. Griesser, T. Andersson and J. Ekman. 2005. Nest predation and habitat change interact to influence Siberian Jay numbers. Oikos 111: 150-158.
Forster, J. A. 1975. Electric fencing to protect sandwich terns against foxes. Biological Conservation 7: 85.
Forest Resources and Management Division. 2016. Land use and land cover of Bhutan 2016: maps and statistics. Forest Resource Management Division, Thimphu, Bhutan.
Ghemiray, D. K. 2016. Assessment of habitat usage by White-bellied Heron (Ardea insignis) at Burichhu nesting site. BSc dissertation, College of Natural Resources, Lobesa, Punakha, Bhutan.
Götmark, F. 1992. The effects of investigator disturbance on nesting birds. Current Ornithology 9: 63-104.
Guppy, M., S. Guppy, R. Marchant, D. Priddel, N. Carlile and P. Fullagar. 2017. Nest predation of woodland birds in south-east Australia: Importance of unexpected predators. Emu-Austral Ornithology 117: 92-96.
Hjertaas, D. G. 1982. Great Blue Herons and Raccoons at Nicolle Flatts. Blue Jay 40: 36-41.
Ikeda, T. 1999. Progress of naturalization of raccoons and related problems in Hokkaido. The Annual Report on Cultural Science, Faculty of Letters, Hokkaido University 47: 149-175.
Iwama, M., K. Yamazaki, M. Matsuyama, Y. Hoshino, M. Hisano, C. Newman and Y. Kaneko. 2017. Masked Palm Civet Paguma larvata summer diet differs between sexes in a suburban area of central Japan. Mammal Study 42: 185-190.
Khandu, P., G. A. Gale and S. Bumrungsri. 2021. Ecological and environmental factors affecting the foraging activity of the White-bellied Heron Ardea insignis (Hume, 1878) in Bhutan. Bird Conservation International 31: 420-437.
Khandu, P., G. A. Gale, R. Pradhan, I. P. Acharja and S. Bumrungsri. 2020a. First record of successful breeding of the critically endangered White-bellied Heron (Ardea insignis) in broadleaved trees. Journal of Animal and Plant Sciences 30: 502-507.
Khandu, P., G. A. Gale, K. Kinley, T. Tandin, S. Shimano and S. Bumrungsri. 2020b. Daily roosting behaviour of the critically endangered White-bellied Heron Ardea insignis as a function of day length. Biological Rhythm Research 53: 812-822.
Kushlan, J. A. 2011. Heron count protocols: inventory, census and monitoring of herons. Heron Conservation. [online]. Accessed 1 May 2021.
Lahti, D. C. 2001. The “edge effect on nest predation” hypothesis after twenty years. Biological Conservation 99: 365-374.
Lenington, S. 1979. Predators and blackbirds: the “uncertainty principle” in field biology. The Auk 96: 190-192.
Major, R. E. and C. E. Kendal. 1996. The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138: 298-307.
Matsunaga, K. 2005. Status report of the Grey Heron in Hokkaido. [online].
Matsunaga, K. 2018. Changes of the nesting sites of Grey Herons (Ardea cinerea) in Hokkaido, northern Japan. Journal of Heron Biology and Conservation 3: 1. [online].
McMahon, B. J., S. Doyle, A. Gray, S. B. A. Kelly and S. M. Redpath. 2020. European bird declines: do we need to rethink approaches to the management of abundant generalist predators? Journal of Applied Ecology 57: 1885-1890.
Mondal, H. S. and G. Maheswaran. 2014. First nesting record of White-bellied Heron Ardea insignis in Namdapha Tiger Reserve, Arunachal Pradesh, India. BirdingASIA 21: 13-17.
Moore, R. P. and W. D. Robinson. 2004. Artificial bird nests, external validity, and bias in ecological field studies. Ecology 85: 1562-1567.
National Center for Hydrology and Meteorology. 2017. Bhutan state of the climate. National Center for Hydrology and Meteorology, Weather and Climate Services Division. Thimphu, Bhutan.
Phillips, T., C. Cooper, J. Dickinson, J. Lowe, R. Rietsma, K. Gifford and R. Bonney. 2007. Nest watch nest monitoring manual. Cornell Lab of Ornithology, Ithaca, New York, U.S.A.
Price, M. R. S. and G. L. Goodman. 2015. White-bellied Heron (Ardea insignis): conservation strategy. IUCN Species Survival Commission White-bellied Heron Working Group, part of the IUCN SSC Heron Specialist Group. [online].
Ricklefs, R. E. 1969. An analysis of nesting mortality in birds. Smithsonian Contributions to Zoology 9: 1-48.
Royal Society for Protection of Nature. 2009. A ray of hope for White-bellied Herons. Rangzhin 2: 2, 4.
Royal Society for Protection of Nature. 2011. The critically endangered White Bellied Heron Ardea insignis. Royal Society for Protection of Nature, Thimphu, Bhutan.
Smith, R. K., A. S. Pullin, G. B. Stewart and W. J. Sutherland. 2010. Effectiveness of predator removal for enhancing bird populations. Conservation Biology 24: 820-829.
White, C. M. and T. L. Thurow. 1985. Reproduction of Ferruginous Hawks exposed to controlled disturbance. The Condor 87: 14-22.
Winton, B. R., D. M. Leslie Jr. and J. R. Rupert. 2000. Breeding ecology and management of snowy plovers in north-central Oklahoma. Journal of Field Ornithology 71: 573-584.
Zanette, L.Y., A. F. White, M. C. Allen and M. Clinchy. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334: 1398-1401.
Zhou, Y., J. Zhang, E. Slade, L. Zhang, F. Palomares, J. Chen, X. Wang and S. Zhang. 2008. Dietary shifts in relation to fruit availability among Masked Palm Civets (Paguma larvata) in central China. Journal of Mammalogy 89: 435-447.



