Food habits of the Malagasy Pond Heron (Ardeola idae) during the breeding season in northern Madagascar
Abstract
We studied the diet of the endangered Malagasy Pond Heron (Ardeola idae) at a monospecific colony in Sofia Lake, in northern Madagascar during one breeding season from November 2018 to March 2019. Two complementary methods were used: 1) the collection of pellets followed by a laboratory analyses and 2) the direct observation of prey taken by the species. Based on 4,062 identified prey items from the 193 pellets, the diet of the Malagasy Pond Heron was composed of invertebrates including insects (81.34%), spiders (4.68%), crustaceans (1.90%), gastropods (0.86%), and vertebrates such as fishes (5.59%), frogs (3.40%) and lizards (2.24%). A large variety of insect families was identified, of which Tenebrionidae (13.47%), Hydrophilidae (11.05%), Acrididae (9.35%), Libellulidae (8.39%) and Dytiscidae (6.92%) were the most dominant. Three species of fish (Cyprinus carpio, Channa sp. and Tilapia sp.) and two species of frogs (Boophis sp. and Rana sp.) were also identified. The main prey items varied significantly through the five months of the breeding season. Coleopterans (Tenebrionidae and Hydrophilidae) were the most abundant group in all months and their consumption was greatest in March. Orthopterans (Acrididae) and Odonatans (Libellulidae) were consumed mainly in November and February, respectively. The consumption of fish was greatest in February and March. Frogs were captured most frequently in February. These results confirm that the Malagasy Pond Heron is a generalist and opportunistic in its feeding habitats. Conservation measures for this endangered heron should not only take place at nesting sites, but also in foraging areas surrounding the nesting colonies.
Key words: feeding habitats; Malagasy Pond Heron; nesting colony; pellet; prey; Sofia Lake.
Introduction
The Malagasy Pond Heron (Ardeola idae) is a migratory wading bird belonging to the Ardeidae family. It breeds in Madagascar, Mayotte and Aldabra (Seychelles), and has a large non-breeding range in East and Central Africa including Kenya, Tanzania, Uganda, Burundi, Rwanda, Zambia, Malawi and Democratic Republic of Congo (Betts 2002, Kushlan and Hancock 2005, Ndang’ang’a and Sande 2008). The species has been classified as globally Endangered since 2004 (IUCN 2020), because of its small remaining population (estimated at 2,000-6,000 individuals in 2000), which is subject to numerous threats such as habitat loss, exploitation of eggs and young and disturbance at nesting sites (Ndang’ang’a and Sande 2008, IUCN 2020).
The Malagasy Pond Heron nests colonially and usually breeds in mixed-species colonies, with Squacco Herons (Ardeola ralloides) and Western Cattle Egrets (Bubulcus ibis) (Burger and Gochfeld 1990, del Hoyo et al. 1992, Kushlan and Hancock 2005). Historically, its main breeding sites were located in the high central plateau, especially around Antananarivo, and in the western wetlands of Madagascar (Ndang’ang’a and Sande 2008). However, in 2015 a discrete monospecific colony was discovered at Sofia Lake in northern Madagascar.
While the distribution, habitat, nesting habits, population trends and breeding bio-ecology of the Malagasy Pond Heron have been well documented (Burger and Gochfeld 1990, del Hoyo et al. 1992, Projet ZICOMA 1999, Kushlan and Hancock 2005, Bunbury 2014, Pruvot et al. 2020, Rabarisoa et al. 2020), little is known about the food habits of this species. Malagasy Pond Herons have occasionally been observed feeding on fish, insects and small invertebrates (Morris and Hawkins 1998), as well as frogs and small reptiles including skinks and geckos (Kushlan and Hancock 2005). Despite the existence of this information, the diet composition and food preference of the endangered Malagasy Pond Heron are still poorly known compared to other heron species in the world. To fill this knowledge gap, this study was conducted with the aim to determine the diet composition and variation of the Malagasy Pond Heron during the breeding season.
Study Area
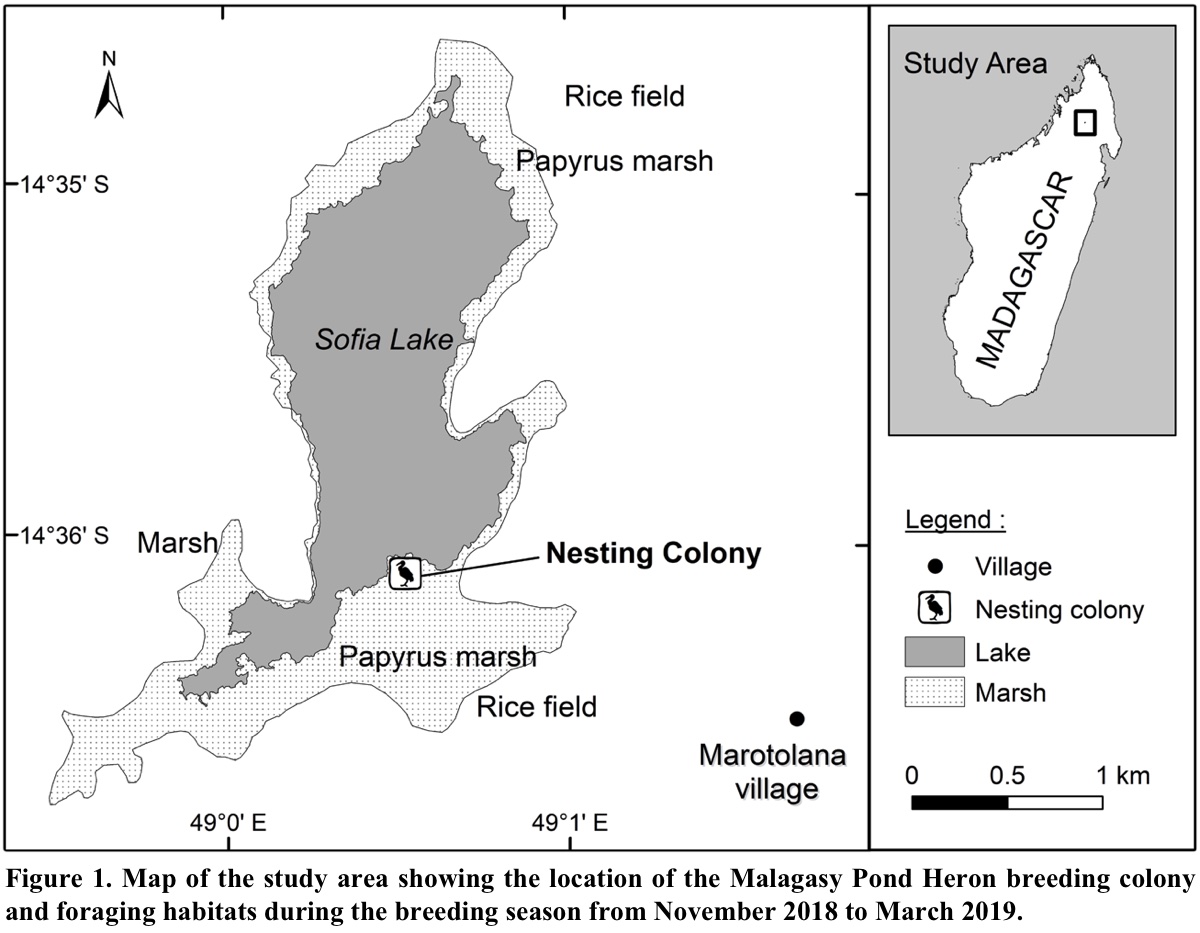
Fieldwork was conducted at Sofia Lake (14° 35′ 04′′ S, 49° 00′ 30′′ E) and surrounding wetlands composed of grasslands, marshes, ponds and ricefields, which are located in the northern highlands of Madagascar, at Marotolana village, District of Bealanana. Sofia Lake with the adjacent wetland ecosystems comprises an area of 1,650 ha and was designated a Ramsar site in May 2017 (Ramsar 2017). It is surrounded by a large cover of emerging aquatic vegetation, which provides habitat for several bird species, including five endemic and threatened species: Malagasy Pond Heron (EN), Meller’s Duck (Anas melleri, EN), Madagascar Grebe (Tachybaptus pelzelnii, EN), Madagascar Rail (Rallus madagascariensis, VU) and Madagascar Snipe (Gallinago macrodactyla, VU). Other heron and egret species such as Western Cattle Egrets, Dimorphic Egrets (Egretta dimorpha), Black Herons (Egretta ardesiaca) and Black-crowned Night Herons (Nycticorax nycticorax) also occur at this site. Despite the existence of other heron species in this study area, the Malagasy Pond Heron formed a monospecific nesting colony during the breeding season (Pruvot et al. 2020).
The breeding colony of the Malagasy Pond Heron was located in the papyrus (Cyperus madagascariensis) marsh vegetation at the south end of Sofia Lake (Fig. 1). The colony has an area of 565 m2 with 102 recorded nests. All nests were placed inside clusters of papyrus 2.5-4.5 m in height (Pruvot et al. 2020). The colony site is surrounded by several types of foraging habitats such as grasslands, marshes, ponds and ricefields. Although the pond herons may feed over a wider area, most individuals preferred foraging in habitat within 5 km from their breeding colony. Therefore, we assumed that all Malagasy Pond Herons observed in the study area were from this colony.
Methods
The diet of the Malagasy Pond Heron was determined using two complementary methods: analysis of regurgitated pellets and direct observation. The analysis of pellets was selected because it is able to supply a representative sample size and has been widely used to determine heron diets (Hafner 1977, Bredin 1983, Si Bachir et al. 2001, Rodriguez et al. 2007, Boukhtache 2008, Roshnath 2015). It is both safe and effective because the digestive system of herons cannot digest some sclerified parts, chitinous exoskeletons and some bones of consumed prey and, therefore, these are regurgitated as pellets. However, this method presents a degree of bias due to the differential digestion rates of each prey item (Kushlan 1978, Marquiss and Leitch 1990, Fasola et al. 1993) and the non-detection of certain consumed prey after their complete digestion (i.e., prey with soft teguments such as earthworm and larvae). For this reason, direct observation of herons feeding in their foraging habitats was also used in the field to complement the pellet data.
Collection and laboratory analyses of pellets
Pellets were collected in the monospecific colony at Sofia Lake during one breeding season from November 2018 to March 2019. The colony area was searched at the middle and at the end of each month (two visits per month), and all visible fresh pellets were collected. As the pellets could not be analysed in the field, a conditioning process was adopted to avoid their damage during the period between collection and analysis in the laboratory. Thus, after each collection, the pellets were subsequently dried in sunlight, measured (length and width) using vernier calipers to the nearest 0.1 mm and weighed using an electronic balance scale. They were then placed in labelled plastic bags with information on the site, date and pellet number. In the laboratory, each pellet was soaked separately for 10-20 min in plastic jars (125 ml) containing water and a few drops of alcohol until soft and pliable. Prey items found were removed from a pellet and sorted. They were grouped together according to their systematic affinities, counted and identified to the lowest taxonomic level possible. Invertebrate remains were identified using determination keys of Borror et al. (1989) with the help of specialists from the laboratory of California Academy of Sciences (CAS) in Madagascar. The identification of fish, amphibians and reptiles was based on the presence of their respective scale traits and bones with the help of literature (Kiener and Richard-Vindard 1972, De Rham 1996, Glaw and Vences 2007). Some doubtful prey remains were identified by referring to the collections in the Zoology Department at the University of Antananarivo. The minimum number of individuals present in each pellet was estimated according to the most commonly found body parts, which represented one individual (heads, mandibles, legs, sclerotised parts, caudal appendages, etc.). Whenever fish remains (e.g., otoliths, pharyngeal bones and chewing pads) were found, the number of individuals was determined by assuming that any detected left- and right-hand side bones of a given species always belonged to the same individual.
Direct observation
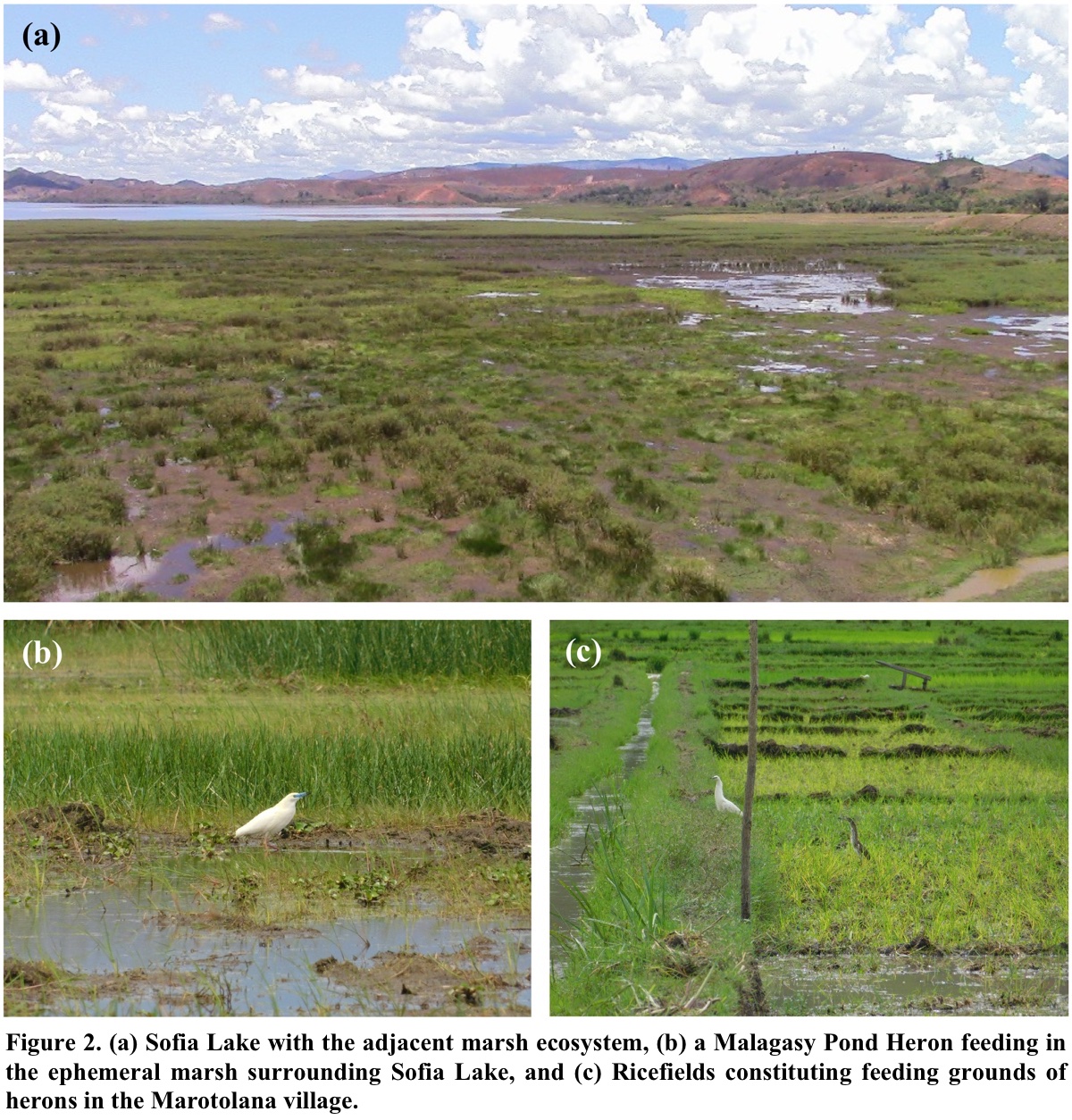
We conducted direct observations of Malagasy Pond Herons on their feeding grounds (Fig. 2), in order to identify as far as possible their captured prey before being swallowed. The observations were made in the feeding habitats in the vicinity of the nesting colony, using binoculars (10 x 50) and spotting scope (20-60 x). They were conducted every week, from November 2018 to March 2019, i.e., a total of 20 observation sessions. Each observation session was conducted for three hours from 0700-1000 h and 1500-1800 h. This method provided data on diet related to specific times and sites but was heavily biased towards situations where birds were easily observed and towards prey that were large or easily identified. Due to this situation, the sizes of all prey were poorly estimated and the identification was not very precise. For slightly larger prey, the identification was only at the group level, such as fish, frog, reptile, insect, dragonfly and worm. Thus, no in-depth data analysis from this method was done; it only served as a confirmation and was supplementary. Indeed, the main advantage of direct observation was the identification of some prey items consumed by the pond herons that were not identified in the analysed pellets.
Data analysis
Data were presented in three ways: (1) number of prey items (NP), (2) prey item percentage (%NP: the ratio of the number of individuals of a given taxon to the total of number of prey items identified) and (3) frequency of occurrence (%FO: the percentage of pellets containing a particular prey taxon) (Rodriguez et al. 2007). The results of pellet measurements and weighing were calculated as mean values and standard deviations. A chi-square test was used to investigate the association of the main prey identified in the pellets and the month of their consumption. We used a G-test (McDonald 2014) to study monthly variation of the main prey items found in the diet, by comparing the number of each prey item during the five month breeding season. All statistical analyses were performed using STATISTICA version 10.0.
Results
A total of 193 pellets was collected and analysed. The pellets of the Malagasy Pond Heron were small and generally dark grey in colour and had an oblong shape measuring 35.84 ± 5.89 mm in length (n = 193, range = 23.2-50.3 mm) and 19.73 ± 1.47 mm wide (n = 193, range = 16.1-23.4 mm) (Fig. 3). The mean dry weight of pellets was 2.20 ± 0.71 g (n = 193, range = 1.2-4.5 g). The mean number of prey recorded in a pellet was 30.07 ± 20.24 (n = 193, range = 8-103 prey items).

Diet composition
A total of 4,062 prey items was identified in the 193 analysed pellets. These prey items were composed of invertebrates with 81.3% of insects (n = 3,304), 4.7% of spiders (n = 190), 1.9% of crustaceans (n = 77), 0.9% of gastropods (n = 35), and vertebrates with 5.6% of fish (n = 227), 3.4% of frogs (n = 138) and 2.2% of small lizards (n = 91). Among the insect prey items, we noted the relative importance of Coleoptera (44.9%), Orthoptera (14.4%), Odonata (10.3%) and Hemiptera (4.9%). A large variety of insect families was also recorded (see Appendix 1). Tenebrionidae (Coleoptera) and Hydrophilidae (Coleoptera), represented 13.5% and 11.1% of the total prey items, respectively, were the main invertebrate groups. These families were followed by Acrididae (Orthoptera, 9.4%), Libellulidae (Odonata, 8.4%), Dytiscidae (Coleoptera, 6.9%) and Naucoridae (Hemiptera, 4.9%) (see also Appendix 1). Three species of fish (Cyprinus carpio, Channa sp. and Tilapia sp.) and two species of frogs (Boophis sp. and Rana sp.) were identified in the diet of the Malagasy Pond Heron. The number and percentage of each identified prey item are given in Appendix 1.
In terms of FO, insects from Coleoptera and Orthoptera were the most recorded prey types. They occurred in 100% of analysed pellets. The Tenebrionidae (FO = 100%) and Acrididae (FO = 100%) were the most important families. The next most frequent groups were Dytiscidae (FO = 77.7%), Hydrophilidae (FO = 72.0%) and Libellulidae (FO = 66.3%). Fish, frogs and lizards occurred, respectively, at 60.6% (n = 117 pellets), 52.9% (n = 102 pellets) and 47.2% (n = 91 pellets) of the total analysed pellets (see Appendix 1).
Temporal variation of diet
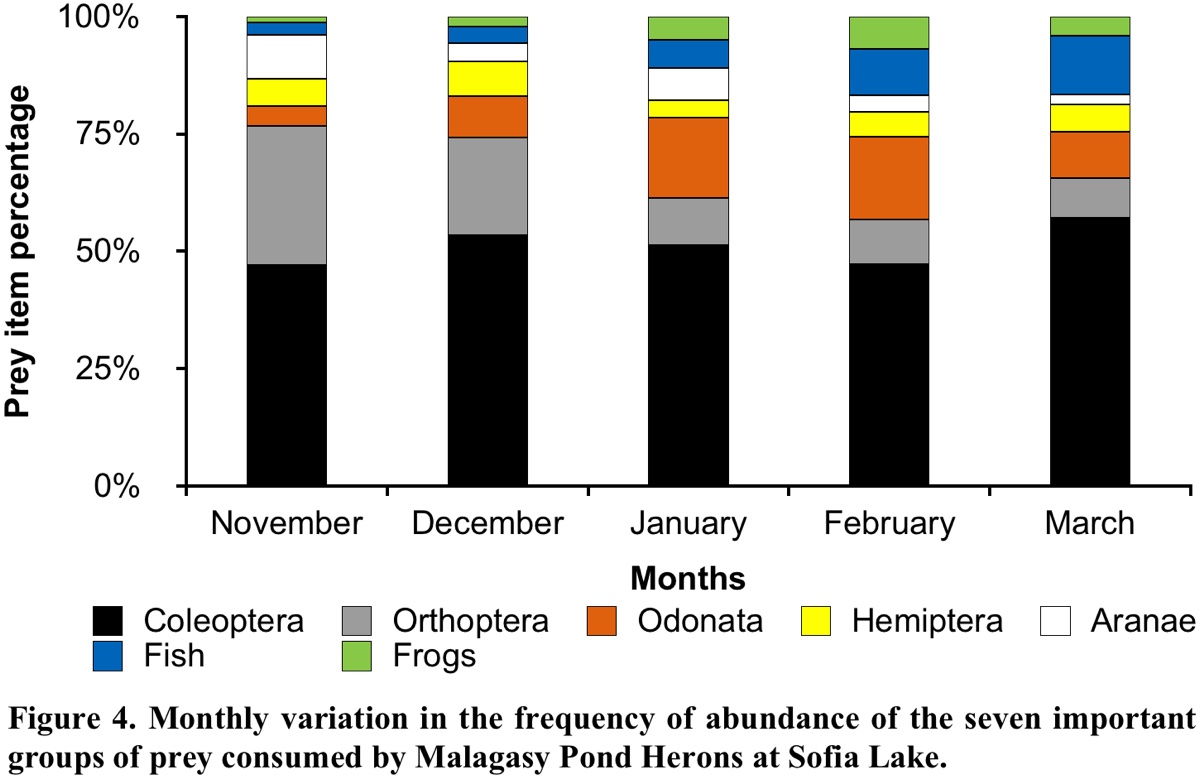
Analysis of the temporal composition of the diet determined seven prey groups. The most abundant in the Malagasy Pond Heron diet composition were: Coleoptera, Orthoptera, Odonata, Hemiptera, spider, fish and frog. We analysed the variation in the %NP of these groups during the five months of the breeding period.
Our results showed a significant dependence between the prey items in the pellets and the months of their consumption (χ224 = 391.23, P < 0.001). Most of the main prey items varied significantly among months, except for Coleoptera (G = 8.48, P = 0.75) with its %NP being almost the same over the five months. However, Coleoptera was the most important group in all months and their consumption was greatest in March (Fig. 4 and Appendix 1). The two main families of this order were Tenebrionidae and Hydrophilidae. The consumption of Orthoptera also varied significantly (G = 138.40, P < 0.001), being greatest in November and least in March. Monthly variation was also observed in the Odonata (G = 86.52, P < 0.001) and Hemiptera (G = 10.42, P = 0.034), with their %NP being greatest in February and December, respectively. The consumption of Aranea (Arachnids) reached maximum values in November and January (G = 40.12, P < 0.001). With regard to vertebrates, fishes were also statistically significant among months (G = 65.97, P < 0.001), and their consumption was greatest in February and March. The consumption of frogs also varied significantly among the five months (G = 40.51, P < 0.001); these prey were more frequently captured in January and February (Fig. 4).
Diet from direct observation
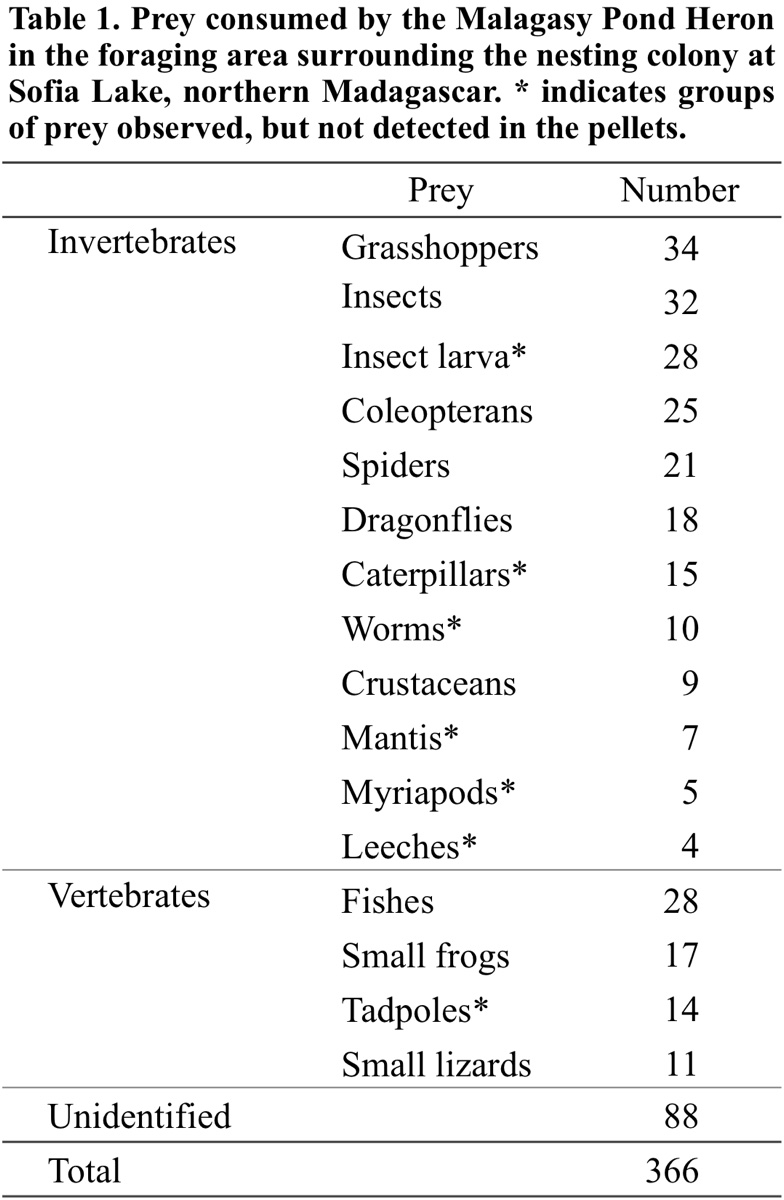
We identified 278 prey items captured by adult and juvenile Malagasy Pond Herons at their foraging sites at Sofia Lake and surrounding areas (Fig. 5). Although the identification of prey was not precise, seven groups of prey were not detected in the analysed pellets and were identified as: mantis, caterpillars, myriapods, worms, insect larvae, leeches and tadpoles (Table 1).

Discussion
This study is the first in Madagascar to analyze in detail the diet of the Malagasy Pond Heron, based on regurgitated pellets and direct observation. However, it has already been anecdotally documented that the Malagasy Pond Heron feeds on fish, frogs, skinks, geckos and insects (Langrand 1995, Morris and Hawkins 1998, Kushlan and Hancock 2005). Our findings did not deviate from those reported, but they show more precisely the different types of prey consumed by this species. Our findings combined with those of previous reports affirm that the Malagasy Pond Heron is a diet generalist feeding on a wide range of invertebrate and vertebrate prey. This diversity of prey constitutes one of the common characteristics for all species belonging to the Ardeidae family (Hancock and Kushlan 1989, Chalabi Belhadj 2008). However, no consumption of mammals and birds was seen for the Malagasy Pond Heron during the study, even though this habit has been reported in some other species of heron such as the Western Cattle Egret, the Black Heron and the Black-crowned Night-Heron (Bredin 1984, Bacha 1999, Kopij 2005, Montesinos et al. 2008). Although there is the possibility this species could eat other types of food that were not detected by our methods, we believe that the Malagasy Pond Heron does not feed on mammals and birds, as is also demonstrated by several herons of the same genus. For example, none of the diet studies of the Squacco Heron carried out worldwide revealed the existence of mammals and birds in their diet (Jazefik 1970, Fasola et al. 1993, Delord et al. 2004, Gautier-Clerc et al. 2004), and similarly for the Indian Pond Heron (Ardeola grayii) which mainly feeds on fish and insects (Sodhi 1992, Santharam 2003, Roshnath 2015).
Furthermore, the basis of the diet could vary according to the ecosystem of a site, food availability and the temporal period. In the Sofia Lake area, insects were the most abundant prey in the diet of the Malagasy Pond Heron during the breeding period. They were represented by 21 families belonging to eight orders, and despite the dominance of insects in the Malagasy Pond Heron diet, we cannot suggest that this species has a food preference towards this class of invertebrates. Indeed, the abundance of each taxon identified in the analysed pellets rather reflects the availability and accessibility of prey in the foraging grounds. This is consistent with the assertion by Bredin (1984) “that there is clearly a close link between diet and the feeding habitats visited by a bird during its annual cycle”. The main foraging habitats of the Malagasy Pond Heron during the breeding period around Sofia Lake were rice fields, grassy marshes and ephemeral ponds, in addition to the lakeshore. The abundance of both land and aquatic insects in these types of habitats is therefore obvious, although we did not study the availability of prey in these habitats. Furthermore, analysis of monthly variations in the diet showed that although insects were the most abundant prey throughout the study period, variations in the %NP of orders and families within this class were observed. Each decrease in one taxon (order or family) was followed by an increase in another. This variation would therefore be related to the variation in the abundance of each prey group on the foraging grounds. The abundance of prey in these feeding grounds depends on the life cycle of prey and the structure of the environment according to the temporal period (water, vegetation, etc.). Similarly, the increase in the consumption of fish and frogs in the diet of the Malagasy Pond Heron observed between January and March could also be explained by the abundance of these prey in the foraging habitats during these months. Indeed, January and March were characterised by abundant rainfall in the study area, which led to the appearance of numerous puddles, marshes, swamps and small ephemeral lakes, which are essential for the development of fish and amphibians.
This study provides new information on the diet and prey composition of the endangered Malagasy Pond Heron. The results are not only an indispensable tool to improve and strengthen conservation programs for this species, but they also reinforce the basic data on Malagasy herons, being one of the least studied bird groups in Madagascar. Although our results showed the importance of insects (especially Coleoptera and Orthoptera) in the diet of the Malagasy Pond Heron, we noted that the species had a wide range of prey types from invertebrates to vertebrates. These results confirm that the Malagasy Pond Heron is a generalist species and opportunistic in its feeding habitats. It was also found that this species had a remarkable ability to adapt to prey variation throughout its breeding period. The varieties of prey and feeding adaptations could certainly be one of the reasons for their reproductive success at Sofia Lake. It is recommended that conservation measures and efforts should focus not only on the nesting habitats, but also on the various foraging habitats within the vicinity of breeding sites. A more detailed study on the diet such as seasonal and yearly variations is needed to increase the understanding of foraging ecology of the Malagasy Pond Heron.
Acknowledgements
This study was supported by The COKETES (Conservation of Keys, Endemic Threatened and Economically Value Species) Project with funding provided by Globally Environment Fund and UN-Environment. Our special thanks go to these institutions. We would like to thank Durrell Wildlife Conservation Trust (DWCT) Madagascar for authorizing access to the study site. We are also grateful to Loukman Kalavah (The Peregrine Fund’s field technician), Médé Rabenosy (DWCT’s technician), Perlin Randrianaivo and Débon Ranaivoson for their assistance in collecting data in the field, and to Balsama Rajemison (California Academy of Science) for her help in identifying insect remains. A special thanks to Russell Thorstrom (The Peregrine Fund, USA) for improving the earlier version of this manuscript.
Literature Cited
Bacha, C. 1999. Contribution à l'étude de la structure et de la variation du régime alimentaire des adultes du Héron garde-boeufs Bubulcus ibis (Linné, 1758) (Aves, Ardeidae) en période de reproduction dans une colonie d'El Kseur (Bejaia, Algérie). Mémoire d’Ingénieur en Ecologie et Environnement, Université de Bejaia, Algérie.
Betts, M. 2002. A systematic list of the birds of Aldabra. African Bird Club Bulletin 9: 32-42.
Borror, D. J., C. A. Triplehorn and N. F. Johnson. 1989. An Introduction Study of Insects, Sixth Edition. Saunders College Publishing, Philadelphia, U.S.A.
Boukhtache, N. 2008. Contribution à l’étude de la niche écologique de la Cigogne blanche Ciconia ciconia L., 1758 (Aves, Ciconiidae) et du Héron garde-bœufs Bubulcus ibis L., 1758 (Aves, Ardeidae) dans la région de Batna. Magister en agronomie, Université El Hadj Lakhdar, Batna, Algérie.
Bredin, D. 1983. Contribution à l'étude écologique de Ardeola ibis (L.): Héron garde-boeufs de Camargue. Thèse de doctorat, Université de Paul Sabatier, Toulouse, France.
Bredin, D. 1984. Régime alimentaire du Héron garde-boeufs à la limite de son expansion géographique récente. Terre et Vie (Revue d’Ecologie) 39: 431-445.
Bunbury, N. 2014. Distribution, seasonality and habitat preferences of the endangered Madagascar pond-heron Ardeola idae on Aldabra Atoll: 2009-2012. Ibis 156: 233-235.
Burger, J. and M. Gochfeld. 1990. Vertical nest stratification in a heronry in Madagascar. Colonial Waterbirds 13: 143-146.
Chalabi Belhadj, G. 2008. Contribution à l’étude des exigences écologiques des Ardeidae et de l’Ibis falcinelle Plegadis falcinellus dans le complexe de zones humides d’El Kala (Algérie). Thèse de doctorat en Sciences agronomiques, Institut National Agronomique d’El Harrach, Alger, Algérie.
del Hoyo, J., A. Elliot and J. Sargatal. 1992. Handbook of the Birds of the World: Ostrich to Ducks, (Vol. 1). Lynx Edicions, Barcelona, Spain.
Delord, K., Y. Kayser, D. Cohez, S. Befeld and H. Hafner. 2004. Fluctuations in the diet of the Squacco Heron Ardeola ralloides in southern France: changes over the last 30 years. Bird Study 51: 69-75.
De Rham, P. H. 1996. Poissons des eaux intérieures de Madagascar. Pp. 423-440 in The Biogéographie de Madagascar (W. R. Lourenço, eds.). ORSTOM, Paris, France.
Fasola, M., P. Rosa and L. Canova. 1993. The diets of the Squacco Herons, Little Egrets, Night, Purple and Grey Herons in their Italian breeding ranges. Revue d’Ecologie (Terre et Vie) 48: 35-47.
Gautier-Clerc, M., Y. Kayser and B. Kiss. 2004. Diet and clutch size of Squacco Heron (Ardeola ralloides) in the Danube Delta (Romania). Annals of the Danube Delta Institue 10: 1-3.
Glaw, F. and M. Vences. 2007. A Field Guide to the Amphibians and Reptiles of Madagascar, Third Edition. Vences and Glaw Verlag, Cologne, Germany.
Hafner, H. 1977. Contribution à l'étude écologique de quatre espèces d'Ardéidés (Egretta garzetta L., Ardeola ralloïdes Scop., Ardeola ibis L., Nycticorax nycticorax L.) pendant leur nidification. Thèse de doctorat, Université de Paul Sabatier, Toulouse, France.
Hancock, J. A. and J. A. Kushlan. 1989. Guide des hérons du monde. Delachaux et Niestlé, Paris, France.
IUCN (International Union for the Conservation of Nature and Natural Resources). 2020. The IUCN Red List of Threatened Species. [online] Accessed 05 June 2021.
Jazefik, M. 1970. Studies on the Squacco Heron, Ardeola ralloides (Scop.). Part IV. Spatial organization and mechanisms integrating the species. Acta Ornithologica 12: 393-443.
Kiener, A. and G. Richard-Vindard. 1972. Fishes of the continental waters of Madagascar. Pp. 477-499 in Biogeography and Ecology in Madagascar (R. Battistini and G. Richard-Vindard, eds.). Springer, The Hague, The Netherlands.
Kopij, G. 2005. Diet of the Cattle Egret Bubulcus ibis in Lesotho. Alauda 73: 457-458.
Kushlan, J. A. 1978. Feeding ecology of wading birds. Pp. 249-297 in Wading Birds (Sprunt, A., J. C. Ogden and S. Winckler, eds.). Research Report No. 7. National Audubon Society, New York, U.S.A.
Kushlan, J. A. and J. A. Hancock. 2005. The Herons. Bird Families of the World 14. Oxford University Press, Oxford, U.K.
Langrand, O. 1995. Guide des Oiseaux de Madagascar. Delachaux et Niestlé, Lausanne, Paris, France.
Marquiss M. and A. F. Leitch. 1990. The diet of Grey Herons (Ardea cinerea) breeding at Loch Leven, Scotland, and the importance of their predation on ducklings. Ibis 132: 535-549.
McDonald, J. H. 2014. G-test of goodness-of-fit. Pp. 53-58 in The Handbook of Biological Statistics (Third edition). Sparky House Publishing, Baltimore, U.S.A.
Montesinos, A., F. Santoul and A. J. Green. 2008. The diet of the night heron and purple heron in the Guadalquivir Marshes. Ardeola 55: 161-167.
Morris, P. and F. Hawkins. 1998. Birds of Madagascar: a Photographic Guide. Pica Press, Robetsbridge, U.K.
Ndang’ang’a, P. K. and E. Sande. 2008. International Single Species Action Plan for the Madagascar Pond-heron (Ardeola idae). CMS Technical Series No. 20, AEWA Technical Series No. 39. Bonn, Germany.
Projet ZICOMA. 1999. Les Zones d’Importance pour la Conservation des Oiseaux à Madagascar. Antananarivo, Madagascar.
Pruvot, Y. Z. M., L. A. Rene de Roland, M. Rakotondratsima, Y. Razafindrakoto, F. Razafindrajao, R. Rabarisoa and R. Thorstrom. 2020. Breeding ecology and nestling growth of the Madagascar Pond Heron (Ardeola idae) in a monospecific colony at Sofia Lake, northern Madagascar. Ostrich 91: 1-13.
Rabarisoa, R., J. Ramanampamonjy, F. Razafindrajao, L. A. Rene de Roland, F. Jeanne, O. Bacar, A. Laubin and F. Bignon. 2020. Status assessment and population of the Madagascar Pond-heron (Ardeola idae) from 1993-2016. Waterbirds 43: 45-54.
Ramsar. 2017. Sofia Lake Madagascar. Fiche descriptive Ramsar. Formulaire FDR créé par le SISR V.1.6 - 29 mai 2017. [online] Accessed 05 June 2021.
Rodriguez, A., B. Rodriguez, B. Rumeu and M. Nogales. 2007. Seasonal diet of the Grey Heron Ardea cinerea on an oceanic island (Tenerife, Canary Islands): indirect interaction with wild seed plants. Acta Ornithologica 42: 72-87.
Roshnath, R. 2015. Preliminary study in diet composition of Indian pond Heron during breeding season. International Journal of Biochemistry and Biotechnology 4: 574-577.
Santharam, V. 2003. Indian pond-herons Ardeola grayii feeding on dragonflies. Journal of Bombay Natural History Society 100: 108.
Si Bachir, A., H. Hafner, Q. J. N. Touren, S. E. Doumindji and S. Lek. 2001. Diet of the adult Cattle Egret (Bubulcus ibis L.) in a new North African colony (Petite Kabylie, Algérie): taxonomic composition and variability. Ardeola 48: 217-223.
Sodhi, N. S. 1992. Feeding ecology of Indian pond heron and its comparison with that of little egret. Pavo 24: 97-112.



